Abstract
IMPORTANCE
Vitamin D (VitD) deficiency is associated with brain structural abnormalities, cognitive decline, and incident dementia.
OBJECTIVE
To assess associations between VitD status and trajectories of change in subdomains of cognitive function in a cohort of ethnically diverse older adults.
DESIGN, SETTING, AND PARTICIPANTS
Longitudinal multiethnic cohort study of 382 participants in an outpatient clinic enrolled between February 2002 and August 2010 with baseline assessment and yearly follow-up visits. Serum 25-hydroxyvitamin D (25-OHD) was measured, with VitD status defined as the following: deficient, less than 12 ng/mL (to convert to nanomoles per liter, multiply by 2.496); insufficient, 12 to less than 20 ng/mL; adequate, 20 to less than 50 ng/mL; or high, 50 ng/mL or higher. Subdomains of cognitive function were assessed using the Spanish and English Neuropsychological Assessment Scales. Associations were evaluated between 25-OHD levels (as continuous and categorical [deficient, insufficient, or adequate]) and trajectories of cognitive decline.
MAIN OUTCOMES AND MEASURES
Serum 25-OHD levels, cognitive function, and associations between 25-OHD levels and trajectories of cognitive decline.
RESULTS
Participants (N = 382 at baseline) had a mean (SD) age of 75.5 (7.0) years; 61.8% were women; and 41.4% were white, 29.6% African American, 25.1% Hispanic, and 3.9% other race/ethnicity. Diagnosis at enrollment included 17.5% with dementia, 32.7% with mild cognitive impairment, and 49.5% cognitively normal. The mean (SD) 25-OHD level was 19.2 (11.7) ng/mL, with 26.2% of participants being VitD deficient and 35.1% insufficient. The mean (SD) 25-OHD levels were significantly lower for African American and Hispanic participants compared with white participants (17.9 [15.8] and 17.2 [8.4] vs 21.7 [10.0] ng/mL, respectively; P < .001 for both). The mean (SD) 25-OHD levels were similarly lower in the dementia group compared with the mild cognitive impairment and cognitively normal groups (16.2 [9.4] vs 20.0 [10.3] and 19.7 [13.1] ng/mL, respectively; P = .006). The mean (SD) follow-up was 4.8 (2.5) years. Rates of decline in episodic memory and executive function among VitD-deficient (episodic memory: β = −0.04 [SE = 0.02], P = .049; executive function: β = −0.05 [SE = 0.02], P = .01) and VitD-insufficient (episodic memory: β = −0.06 [SE = 0.02], P < .001; executive function: β = −0.04 [SE = 0.02], P = .008) participants were greater than those with adequate status after controlling for age, sex, education, ethnicity, body mass index, season of blood draw, vascular risk, and apolipoprotein E4 genotype. Vitamin D status was not significantly associated with decline in semantic memory or visuospatial ability. Exclusion of participants with dementia did not substantially affect the associations between VitD status and rates of cognitive decline.
CONCLUSIONS AND RELEVANCE
Low VitD status was associated with accelerated decline in cognitive function domains in ethnically diverse older adults, including African American and Hispanic individuals who exhibited a high prevalence of VitD insufficiency or deficiency. It remains to be determined whether VitD supplementation slows cognitive decline.
Vitamin D (VitD) deficiency and insufficiency, defined as a serum 25-hydroxyvitamin D (25-OHD) level less than 12 ng/mL (to convert to nanomoles per liter, multiply by 2.496) and between 12 and 20 ng/mL, respectively,1 are highly prevalent in the United States. The National Health and Nutrition Examination Survey found the prevalence of combined deficiency and insufficiency among the general adult population to be 42%, with even higher prevalence among Hispanic (69%) and African American (82%) individuals.2 In addition to promoting calcium absorption and bone health, it is now recognized that VitD may influence all organ systems. Both the VitD receptor (VDR) and the enzyme that converts 25-OHD to the active form of the vitamin, 1,25-dihydroxyvitamin D, are expressed in all human organs, including the brain.3,4 Thus, research has been increasingly examining the associations between VitD status and a variety of health outcomes, including dementia and age-associated cognitive decline.
Studies examining the effect of VitD insufficiency on cognitive decline with age are motivated by the approximately 50% prevalence of hypovitaminosis in those older than 65 years, which increases to 70% to 90% among cognitively impaired individuals.5,6 Moreover, epidemiological evidence indicates that low VitD levels in blood are associated with increased risk of incident Alzheimer disease (AD) and dementia. For example, in the Cardiovascular Health Study, VitD deficiency7 was associated with hazard ratios for incident AD and all-cause dementia of approximately 2.2 to 2.3. Evidence also exists that the overall risk for incident dementia is proportional to baseline VitD level.8 Consistent with increased dementia risk, VitD levels are also reduced in individuals with mild cognitive impairment (MCI)9 and are inversely associated with cognitive performance, particularly on executive function tests.10-12 Furthermore, reduced VitD levels are associated with vascular risk factors such as hypertension and cardiovascular disease, which are also associated with dementia.13-16 These data offer evidence that hypovitaminosis D may contribute to cognitive decline and late-life dementia risk, although the exact mechanism is uncertain.
Most of the studies discussed were conducted in predominantly white cohorts and thus do not fully reflect the impact of hypovitaminosis D on the general population, as individuals of other races/ethnicities are more likely to have low VitD status.2 Therefore, we investigated the associations between hypovitaminosis D and cognitive decline in an ethnically diverse cohort of older adults.
Methods
Participant Sample
The sample consisted of 382 participants in the University of California at Davis Alzheimer's Disease Center longitudinal community diversity study, enrolled between February 2002 and August 2010, which researches risk factors for cognitive decline and transition to dementia within a highly diverse cohort.17 Of the 382 individuals, a subset (n = 318 [83.2%]) had at least 2 cognitive evaluations (range, 2-11) allowing for longitudinal evaluation. Recruitment into the study was through 2 routes as described previously17: (1) 72.2% of participants were recruited through community-based outreach designed to enhance racial/ethnic diversity and the spectrum of cognitive dysfunction, with an emphasis on normal cognition and MCI; and (2) the remaining 27.8% were recruited through memory clinic referrals. The overall sample included 113 African American participants (29.6%), 96 Hispanic participants (25.1%), 158 white participants (41.4%), and 15 participants from other racial/ethnic groups (3.9%; 6 Asian, 6 Filipino, 3 other).
Regardless of recruitment source, inclusion criteria were being older than 60 years, having the ability to speak English or Spanish, and being community dwelling. No participant was institutionalized during the observational period of this study. Exclusion criteria included physical or psychosocial issues that would prevent longitudinal assessment such as unstable major medical illness (eg, cancer with poor prognosis), major primary psychiatric disorder (schizophrenia, bipolar disorder, or recurrent major depressive disorder), or substance abuse or dependence within 5 years. All participants provided written informed consent, and the study was approved by the institutional review boards at the University of California at Davis, the VA Northern California Health Care System, and San Joaquin General Hospital.
Clinical Evaluations
All participants received multidisciplinary diagnostic evaluations through the University of California at Davis Alzheimer's Disease Center at baseline and at approximately annual follow-ups. Baseline and follow-up evaluations, which included detailed medical history, neurological examination, and neuropsychological testing, were performed according to previously described methods prescribed by the National Alzheimer's Coordinating Center Uniform Data Set.17-19 A physician fluent in Spanish examined Spanish-monolingual participants (approximately 19% of the Hispanic group). An informant with close contact with the participant was interviewed about level of independent functioning. Clinical evaluations included standardized neuropsychological tests performed at baseline and at follow-up.19 Importantly, all clinical tests and diagnoses were distinct from the outcome measures used in the analyses (ie, cognitive test scores). Routine dementia workup laboratory tests were obtained at the baseline evaluation for all participants and when clinically indicated at follow-up evaluations. Body mass index (BMI) was determined from baseline height and weight (calculated as weight in kilograms divided by height in meters squared).
The multidisciplinary clinical assessment included physical and neurological examination, the Clinical Dementia Rating,20 neuroimaging, laboratory work, and neuropsychological testing from the Uniform Data Set Neuropsychological Battery.19 Dementia was diagnosed using Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised)21 criteria with the modification that dementia could be diagnosed without memory impairment if there was impairment within 2 or more other cognitive domains. Mild cognitive impairment was diagnosed according to standard criteria and was further subtyped according to current Uniform Data Set guidelines.18 Normal cognition was diagnosed if there was no clinically significant cognitive impairment. All diagnoses were made blind to research neuropsychological testing and VitD status. This approach has proven validity to correctly diagnose clinical MCI and dementia in our study population that includes Hispanic and African American individuals.22,23
Neuropsychological Outcome Measures
The primary cognitive outcome measures in this study were derived from the Spanish and English Neuropsychological Assessment Scales administered at all evaluations. These scales have undergone extensive development and psychometric assessment as a cognitive battery relevant to diseases of aging.22,24-26 This study used a subset of Spanish and English Neuropsychological Assessment Scales tests to measure 4 cognitive domains: episodic memory, semantic memory, visual perception, and executive function. Episodic memory was a composite verbal episodic memory score derived from a multitrial word list learning test (word list learning).25 Semantic memory was a composite of highly correlated verbal (object naming) and nonverbal (picture association) tasks. Spatial localization assesses the ability to accurately perceive and reproduce spatial relationships. Executive function was a composite measure constructed from component tasks of category fluency, phonemic (letter) fluency, and working memory (digit span backward, visual span backward, list sorting).
Three alternate word list forms matched for list structure were used for the episodic memory measure. They were alternated in a rotating sequence (1-2-3) across the annual longitudinal evaluations to reduce practice effects. Potential form differences were accounted for in longitudinal analyses of episodic memory.
Assessment of Vascular Risk Factors
The presence or absence of various vascular risk factors and conditions was based on thorough review of the participant's medical history, medical records, and medications at both the initial and follow-up visits. Vascular risk was defined as the percentage of total possible risk factors of hypertension, hypercholesterolemia, and diabetes mellitus.
Acquisition of Blood and VitD Levels
Nonfasting blood samples were collected by standard venipuncture. Samples were collected in serum separator tubes and allowed to clot at room temperature for 0.5 to 1 hour and then placed on ice until centrifugation and collection of serum within 4 hours of the draw. Serum was then stored at −80°C until analysis. The 25-OHD concentrations were determined by competitive immunoassay. In addition to continuous measures of 25-OHD level, individuals were assigned to a categorical status based on current Institute of Medicine guidelines1: deficiency was indicated by a serum 25-OHD level less than 12 ng/mL; insufficiency, 12 to less than 20 ng/mL; adequacy, 20 to less than 50 ng/mL; and high, 50 ng/mL or higher. Individuals with high 25-OHD levels (n = 5) were excluded from the cognitive analyses.
Statistical Analysis
Vitamin D, characterized by continuous 25-OHD levels and a categorical variable (VitD status), was the primary predictor of interest. Due to skewness, 25-OHD levels were natural log transformed. Analysis of variance or χ2 tests (or Fisher exact tests) were used to compare baseline 25-OHD levels or VitD status, respectively, by season of blood draw, cognitive diagnosis, and race/ethnicity in the full cohort (n = 382). Post hoc pairwise comparisons, adjusted for multiple comparisons using the Tukey honestly significant difference approach (for 25-OHD) or Bonferroni correction (for VitD status), were conducted if an overall difference by groups was detected. Next, linear regression was used to assess the association between VitD level or status and baseline cognitive function (n = 382). Finally, repeated-measures models, with random intercepts and slopes to account for between-person differences in starting level and rate of change, were used to assess associations between VitD and annualized change in cognition since baseline in the subset with longitudinal assessment (n = 318). For the cross-sectional and longitudinal analyses, participants with high VitD (n = 5) were removed because there were too few individuals to evaluate for cognitive outcomes. The remaining 59 participants were excluded from the longitudinal analysis because they did not have sufficient follow-up owing to progression to severe dementia, dropout, loss to follow-up, and death. These 59 participants were older, had more vascular risk factors, and were more likely to have dementia at baseline compared with the rest of the cohort. Unadjusted models and models adjusted for demographic characteristics, BMI, season of blood draw, vascular risk, and presence of at least 1 apolipoprotein E4 (ApoE4) allele were fit for each of the 4 cognitive domains assessed. Assumptions of the models were checked and met by the data. Secondary analyses assessed associations between VitD and cognitive decline in participants without dementia at baseline. All analyses were conducted in SAS version 9.3 statistical software (SAS Institute, Inc), and P < .05 was considered statistically significant.
Results
Demographic Characteristics
Baseline characteristics of the study cohort are summarized in Table 1. The mean (SD) age was 75.5 (7.0) years, and 61.8% were women. The cohort was racially and ethnically diverse, with African American and Hispanic individuals accounting for 54.7% of the participants. Approximately 90% of Hispanic participants in the study were of North, Central, or South American ancestry, most commonly Mexico. The mean educational level obtained was about equivalent to high school graduation (mean [SD], 12.7 [4.4] years). On average, the cohort was overweight (mean BMI, >25) and had 2 vascular risk factors (hypertension and hypercholesterolemia were most common). Among the participants, 36.7% were ApoE4 allele carriers (≥1 E4 allele). In the cohort, 49.5% were cognitively normal, 32.7% had MCI, and 17.5% had dementia.
Table 1.
Characteristics of Study Participants by Race/Ethnicitya
| Characteristic | Race/Ethnicity | ||||
|---|---|---|---|---|---|
| White | African American | Hispanic | Otherb | P Value | |
| Participants, No. (%) | 158 (41.4) | 113 (29.6) | 96 (25.1) | 15 (3.9) | |
| Age, mean (SD), y | 76.6 (7.2) | 75.0 (6.6) | 74.1 (6.9) | 77.4 (5.9) | .02 |
| Women, No. (%) | 82 (51.9) | 79 (69.9) | 64 (66.7) | 11 (73.3) | .009 |
| Education, mean (SD), y | 14.5 (3.1) | 13.1 (3.1) | 9.2 (5.4) | 11.8 (3.3) | <.001 |
| BMI, mean (SD) | 27.0 (5.4) | 29.6 (5.9) | 28.8 (5.6) | 24.5 (5.3) | <.001 |
| Vascular risk score, mean (SD) | 0.43 (0.33) | 0.56 (0.36) | 0.56 (0.38) | 0.40 (0.32) | .005 |
| ≥1 Apolipoprotein E4 allele, No. (%) | 65 (42.5) | 48 (43.2) | 23 (24.2) | 1 (7.1) | .001 |
| Cognitive diagnosis, No. (%) | |||||
| Cognitively normal | 65 (41.1) | 60 (53.1) | 54 (56.2) | 10 (66.7) | .03 |
| MCI | 64 (40.5) | 37 (32.7) | 22 (22.9) | 2 (13.3) | |
| Dementia | 29 (18.3) | 15 (13.3) | 20 (20.8) | 3 (20.0) | |
| Unknown | 0 | 1 (0.9) | 0 | 0 | |
| Serum 25-OHD level, mean (SD), ng/mL | 21.7 (10.0) | 17.9 (15.8) | 17.2 (8.4) | 16.0 (5.7) | <.001 |
| Vitamin D status, No. (%)c | |||||
| Deficient, <12 ng/mL | 21 (13.3) | 48 (42.5) | 27 (28.1) | 4 (26.7) | <.001 |
| Insufficient, 12 to <20 ng/mL | 57 (36.1) | 31 (27.4) | 40 (41.7) | 6 (40.0) | |
| Adequate, 20 to <50 ng/mL | 78 (49.4) | 31 (27.4) | 29 (30.2) | 5 (33.3) | |
| High, ≥50 ng/mL | 2 (1.3) | 3 (2.6) | 0 | 0 | |
| Duration of follow-up, mean (SD), yd | 4.7 (2.7) | 4.8 (2.4) | 5.0 (2.5) | 4.8 (2.4) | .90 |
| Follow-ups, mean (SD), No.d | 5.1 (2.4) | 4.9 (2.0) | 5.0 (2.1) | 4.9 (2.1) | .87 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MCI, mild cognitive impairment; 25-OHD, 25-hydroxyvitamin D.
SI conversion factor: To convert 25-OHD to nanomoles per liter, multiply by 2.496.
Missing data: BMI, n = 19; vascular risk score, n = 22; and at least 1 apolipoprotein E4 allele, n = 9.
American Indian/Alaskan Native, n = 1; Asian, n = 6; Filipino, n = 6; and unknown, n = 2.
Vitamin D status categories as defined by the Institute of Medicine based on serum 25-OHD level.1
Longitudinal data were available for 133 white participants, 90 African American participants, 84 Hispanic participants, and 11 participants of other race/ethnicity.
Factors Influencing Baseline VitD Levels
Average baseline VitD levels were below national standards for adequacy for the entire cohort, with a mean (SD) serum 25-OHD concentration of 19.2 (11.7) ng/mL. The VitD status was similarly low; 61.3% of the participants were either deficient (<12 ng/mL; 26.2% of participants) or insufficient (12 to <20 ng/mL; 35.1% of participants). Neither VitD status nor prevalence of deficiency differed significantly by season of blood draw (P = .24 and .35, respectively) (eTable in the Supplement).
As expected,2 mean 25-OHD levels varied by race and ethnicity (P < .001). The mean (SD) 25-OHD level was higher in white participants (21.7 [10.0] ng/mL) than among African American participants (17.9 [15.8] ng/mL) and Hispanic participants (17.2 [8.4] ng/mL) (adjusted P < .001) (Table 1). Prevalence of VitD deficiency also differed by race/ethnicity (P < .001), with rates lower in white participants (13.3%) than in Hispanic (28.1%) and African American (42.5%) participants (Table 1).
The baseline mean (SD) 25-OHD level was lower in individuals with dementia (16.2 [9.4] ng/mL) compared with cognitively normal individuals (19.7 [13.1] ng/mL) and those with MCI (20.0 [10.3] ng/mL) (P = .006) (Table 2). Similarly, the prevalence of VitD deficiency and insufficiency were higher in the dementia group (35.8% and 40.3%, respectively) compared with the cognitively normal group (24.3% and 34.9%, respectively) and the MCI group (24.0% and 32.8%, respectively), but these differences did not reach statistical significance (P = .12). These differences do not appear to reflect differences in general nutritional status, as the mean (SD) BMI of participants with dementia (27.4 [4.9]) did not differ from cognitively normal participants (29.2 [6.4]) or those with MCI (26.8 [4.8]), although the BMI in participants with MCI was slightly lower than in the cognitively normal group (adjusted P = .002). There was no interaction between cognitive status, BMI, and race/ethnicity (P = .41).
Table 2.
Vitamin D Status by Cognitive Diagnosis
| Vitamin D Level | Cognitive Diagnosis | ||
|---|---|---|---|
| Cognitively Normal (n = 189) | MCI (n = 125) | Dementia (n = 67) | |
| Serum 25-OHD level, mean (SD), ng/mL | 19.7 (13.1) | 20.0 (10.3) | 16.2 (9.4)a |
| Vitamin D status, No. (%)b | |||
| Deficient, <12 ng/mL | 46 (24.3) | 30 (24.0) | 24 (35.8) |
| Insufficient, 12 to <20 ng/mL | 66 (34.9) | 41 (32.8) | 27 (40.3) |
| Adequate, 20 to <50 ng/mL | 74 (39.2) | 53 (42.4) | 15 (22.4) |
| High, ≥50 ng/mL | 3 (1.6) | 1 (0.8) | 1 (1.5) |
Abbreviations: MCI, mild cognitive impairment; 25-OHD, 25-hydroxyvitamin D.
SI conversion factor: To convert 25-OHD to nanomoles per liter, multiply by 2.496.
Mean of 25-OHD level in the dementia group was significantly lower than the cognitively normal and MCI groups. Data were analyzed on the logarithmic scale: overall group difference, P = .006; pairwise differences between the dementia group and the other 2 groups were significant after Tukey honestly significant difference correction for multiple comparisons (P < .05).
Vitamin D status categories as defined by the Institute of Medicine based on serum 25-OHD level.1
VitD Levels and Cross-sectional Cognitive Performance
Cross-sectional associations between VitD status and cognitive performance by domain are shown in Table 3. Controlling for age, sex, race/ethnicity, education, BMI, season of draw, vascular risk, and ApoE4 genotype, no significant correlations were observed between log 25-OHD concentration and any of the cognitive domains scored when VitD levels were treated as a continuous measure. However, when 25-OHD level was stratified into groups, individuals with VitD deficiency showed significantly lower baseline semantic memory (P = .02), visuospatial ability (P = .04), and executive function (P = .01) scores relative to those with adequate levels. Baseline episodic memory performance was not associated with VitD deficiency. No significant differences in baseline cognitive performance were seen when comparing those with adequate levels vs those with insufficiency.
Table 3.
Associations Between Vitamin D Status and Baseline Cognitive Function Scores
| Vitamin D Level | β (SE) | |||
|---|---|---|---|---|
| Episodic Memory (n = 325) | Semantic Memory (n = 324) | Visuospatial Ability (n = 288) | Executive Function (n = 329) | |
| Log serum 25-OHD levela | 0.10 (0.11) | 0.17 (0.09) | 0.12 (0.12) | 0.11 (0.08) |
| Vitamin D statusb | ||||
| Deficient, <12 ng/mL | –0.23 (0.13) | –0.25 (0.11)c | –0.31 (0.15)c | –0.25 (0.10)c |
| Insufficient, 12 to <20 ng/mL | –0.04 (0.11) | –0.05 (0.09) | –0.02 (0.12) | –0.002 (0.08) |
| Adequate, 20 to <50 ng/mL | [Reference] | [Reference] | [Reference] | [Reference] |
Abbreviation: 25-OHD, 25-hydroxyvitamin D.
SI conversion factor: To convert 25-OHD to nanomoles per liter, multiply by 2.496.
Multiple regression coefficients (β [SE]) between log serum 25-OHD level and cognitive function scores were obtained controlling for age, sex, race/ethnicity, education, body mass index, season of blood draw, vascular risk score, and apolipoprotein E4 genotype. Sample sizes represent individuals with complete data for serum 25-OHD level, the specified cognitive function test, and all the covariates. No significant associations between log serum 25-OHD level and any of the cognitive function tests were found.
Vitamin D status categories as defined by the Institute of Medicine based on serum 25-OHD level.1 Coefficients (β [SE]) represent the difference between the particular group and the adequate group (reference group), assuming the values of the covariates are held constant.
Significantly lower than the adequate group (P < .05).
VitD Levels and Longitudinal Cognitive Performance
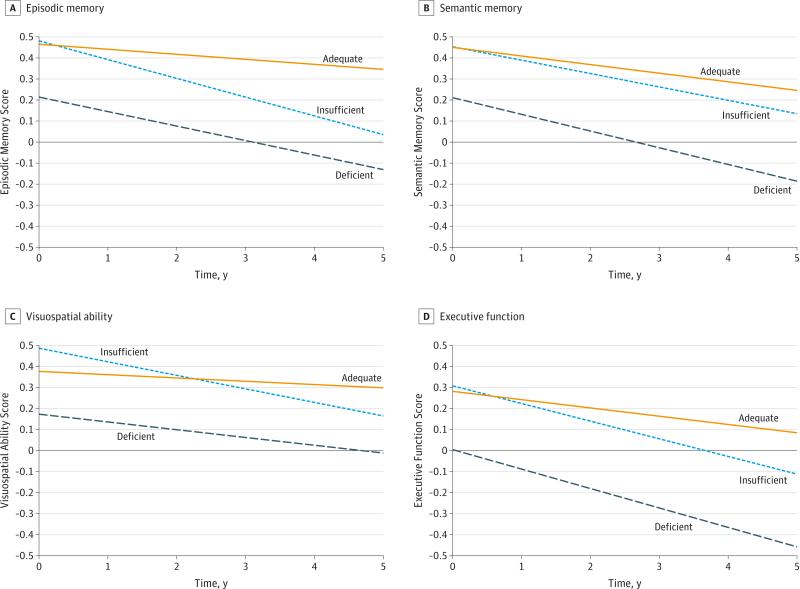
A subset of individuals (n = 318) had at least 1 follow-up cognitive assessment (mean [SD] number of assessments, 5 [2]; mean [SD] follow-up period, 4.8 [2.5] years); 55% had more than 5 cognitive assessments. Associations between VitD and change in cognitive domain scores are shown in Table 4. Controlling for age, sex, race/ethnicity, education, BMI, season of draw, vascular risk, and ApoE4 genotype and considering VitD levels as a continuous measure, a 1-unit increase in natural log 25-OHD concentration was associated with significantly slower rates of decline in episodic memory (P = .01), semantic memory (P = .04), and executive function (P = .001). In particular, the rate of decline for a participant at a midnormal level of 35 ng/mL was 0.022 SD/year in episodic memory, 0.028 SD/year in semantic memory, and 0.027 SD/year in executive function, and for every 50% reduction in 25-OHD concentration, the rate of decline increased by 0.035 SD/year in episodic memory, 0.028 SD/year in semantic memory, and 0.042 SD/year in executive function. When considering VitD status subgroups, rates of cognitive decline were greater in the VitD-deficient and VitD-insufficient individuals than those with adequate status for episodic memory (β = −0.04 [SE = 0.02], P = .049; and β = −0.06 [SE = 0.02], P < .001, respectively) and executive function (β = −0.05 [SE = 0.02], P = .01; and β = −0.04 [SE = 0.02], P = .008, respectively). During 5 years of follow-up, VitD-deficient individuals declined 0.30 SD (0.06 SD/year) in episodic memory and 0.46 SD (0.08 SD/year) in executive function, while those with a normal 25-OHD status declined 0.10 SD (0.02 SD/year) in episodic memory and 0.20 SD (0.04 SD/year) in executive function (Figure). Differences in rates of decline were not significant for visuospatial ability (P = .06). There were no significant group differences in rates of decline for semantic memory (P = .15). These findings are not due to differences in the prevalence of dementia by VitD status as the results were similar even after removing participants with dementia at baseline.
Table 4.
Associations Between Vitamin D Status and Longitudinal Change in Cognitive Function
| Model | β (SE) | |||
|---|---|---|---|---|
| Episodic Memory (n = 264) | Semantic Memory (n = 274) | Visuospatial Ability (n = 226) | Executive Function (n = 274) | |
| Unadjusteda | ||||
| Model 1, continuous vitamin D level | ||||
| Time | –0.06 (0.01)b | –0.06 (0.01)b | –0.02 (0.01)b | –0.08 (0.01)b |
| Log serum 25-OHD level × time | 0.01 (0.02) | –0.02 (0.02) | 0.002 (0.02) | 0.006 (0.02) |
| Model 2, categorical vitamin D status | ||||
| Time | –0.05 (0.01)b | –0.07 (0.01)b | –0.01 (0.01) | –0.07 (0.01)b |
| Vitamin D statusc | ||||
| Deficient × time | –0.003 (0.02) | 0.02 (0.02) | 0.01 (0.02) | 0.004 (0.02) |
| Insufficient × time | –0.05 (0.02)d | 0.001 (0.02) | –0.03 (0.02) | –0.02 (0.02) |
| Adequate × time | [Reference] | [Reference] | [Reference] | [Reference] |
| Adjustedd | ||||
| Model 1, continuous vitamin D level | ||||
| Time | –0.05 (0.02)b | –0.05 (0.02)b | –0.04 (0.03) | –0.06 (0.02)b |
| Log serum 25-OHD level × time | 0.05 (0.02)b | 0.04 (0.02)b | 0.04 (0.02) | 0.06 (0.02)b |
| Model 2, categorical vitamin D status | ||||
| Time | –0.02 (0.02) | –0.04 (0.02) | –0.02 (0.03) | –0.04 (0.02) |
| Vitamin D statusc | ||||
| Deficient × time | –0.04 (0.02)b | –0.04 (0.02) | –0.02 (0.03) | –0.05 (0.02)b |
| Insufficient × time | –0.06 (0.02)b | –0.02 (0.02) | –0.05 (0.02) | –0.04 (0.02)b |
| Adequate × time | [Reference] | [Reference] | [Reference] | [Reference] |
Abbreviation: 25-OHD, serum 25-hydroxyvitamin D.
Coefficients (β [SE]) for average change over time for an individual with a serum 25-OHD level of 20 ng/mL (lower bound of adequate group) and association between a 1-unit difference on log serum 25-OHD levels and change in cognitive function, or average change over time for an individual with a vitamin D level in the adequate range and the average difference in rate of change associated with having deficient or insufficient vitamin D status.
P < .05.
Vitamin D status categories as defined by the Institute of Medicine based on serum 25-OHD level.1 Deficient indicates less than 12 ng/mL; insufficient, 12 to less than 20 ng/mL; and adequate, 20 to less than 50 ng/mL (to convert to nanomoles per liter, multiply by 2.496).
Coefficients (β [SE]) from the model controlling for season of blood draw (reference = winter), age (centered at 65 years), sex (reference = female), race/ethnicity (reference = white), education (centered at 12 years), body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]; centered at 25), vascular risk score, and apolipoprotein E4 genotype (reference = 0 E4 alleles). Coefficients are interpreted as the average change over time for a 65-year-old white woman with a serum 25-OHD level of 20 ng/mL (lower bound of adequate), 12 years of education, no apolipoprotein E4 alleles, no vascular risk factors, and a BMI of 25; the difference in change associated with a 1-unit increase on log serum 25-OHD level or the average annual change for a 65-year-old white woman with a vitamin D level in the adequate range, 12 years of education, no apolipoprotein E4 alleles, no vascular risk factors, and a BMI of 25; and the average difference in rate of change associated with having deficient or insufficient vitamin D status.
Figure. Trajectories of Cognitive Function Domains Over Time by Baseline Vitamin D Status.
Trajectories across measured cognitive domains are shown for those with adequate, insufficient, and deficient vitamin D levels at baseline for episodic memory (A), semantic memory (B), visuospatial ability (C), and executive function (D). Slopes of decline differed significantly from adequate for both insufficient and deficient levels for episodic memory (P < .001 and P = .049, respectively) and executive function (P = .008 and P = .01, respectively) after adjustment for covariates.
Discussion
Our study confirms previous cross-sectional findings showing significant differences in VitD levels among individuals with normal cognition, MCI, and dementia.7,9,27 In addition, we confirmed the expected difference in levels of VitD according to race/ethnicity among our multiethnic cohort.2 While cross-sectional differences in cognitive ability were only weakly associated with VitD, we found significant differences in trajectories of cognitive performance in association with VitD, whether treated as a continuous or categorical variable. Moreover, the rate of cognitive decline associated with hypovitaminosis D (insufficiency and deficiency) was greatest for episodic memory and executive function, the 2 cognitive domains strongly associated with AD dementia. This effect was still present when participants with dementia were removed from the analysis. The magnitude of the effect of VitD insufficiency on cognition was substantial. For example, in the adjusted model, VitD insufficiency was associated with a reduction of 0.08 SD/ year in episodic memory performance, a level of decline comparable with amnestic MCI groups and nearly 3 times greater than in individuals who remained cognitively normal during 3 years of observation as previously shown.23 Adjusting for common covariates known to influence cognition, such as vascular risk, did not modify this relationship.28-30 Adjusting for BMI, which is associated with increased vascular risk, cognitive decline,31 and lower VitD levels,2 also did not alter these relationships. In addition, differences in cognitive trajectories attributed to VitD status cannot be explained by differences in the proportion of race/ethnicity among the various categories, as we have previously shown that longitudinal change in cognitive performance is not influenced by race/ethnicity after adjusting for differences in age, education, sex, and baseline diagnosis.32 Therefore, our results extend current knowledge regarding the effect of hypovitaminosis D on cognitive function and underscore the relevance of identifying high-risk populations with low VitD status.
These findings are relevant to changing US demographic characteristics.33 During the 2011 census, 13% of the population classified themselves as black and nearly 17% as Hispanic, and these percentages are expected to increase substantially.34 Risk factors for dementia also are known to differ according to race and ethnicity. For example, vascular disease and other nonamyloid disorders may have a greater effect on cognition among Hispanic and African American individuals.13,14,35,36 Our data suggest that hypovitaminosis D may be another risk factor for dementia among individuals of nonwhite race/ethnicity.
Skin pigmentation serves as an important protective factor for skin cancer in sunny climates37 but may conversely be a risk factor for hypovitaminosis D for those in less sunny climates or who spend little time outdoors. Exposure of the skin to sunlight is the major source of VitD.38 Because the melanin in skin pigmentation absorbs sunlight in wavelengths important to the conversion of 7-dehydrocholesterol into pre-VitD,39 individuals of nonwhite race/ethnicity are more likely to have VitD insufficiency than white individuals of northern or central European ancestry.2 Hypovitaminosis D not only is relevant to cognition but may also promote other health risks such as hypertension40,41 and cardiovascular disease,42 2 diseases particularly prevalent among African American and Hispanic individuals and associated with increased dementia risk.43 However, recent evidence from the Copenhagen Heart Study suggests that VitD deficiency may be specific to AD risk, as vascular dementia was not associated with low VitD status.44
Diet is the other major source of VitD. Dietary VitD is obtained particularly through dairy consumption.2 National Health and Nutrition Examination Survey data45 showed that dietary intake of dairy products was below recommended levels for most individuals (nearly 80%), but particularly low among minority groups with only 6.5% of African American individuals and 11.4% of Mexican American individuals consuming the recommended 3 daily servings of dairy products. Unfortunately, we did not have dietary intake data on our cohort.
Although the biological mechanisms associating dementia risk with low VitD levels are not fully understood, low VitD status has been associated with increased white matter hyperintensities46,47 and enlarged ventricular volumes.48 Serum 25-OHD is also associated with reduced β-amyloid 1-42 concentrations in cerebrospinal fluid,47 a recognized risk factor for incident dementia.49 Additional support for the role of VitD insufficiency in AD risk comes from genetic studies of VDR polymorphisms. Genetic linkage to late-onset AD has been assigned to chromosome 12q13, which includes the gene for VDR.50 Subsequent assessment of specific polymorphisms in VDR, in particular those designated as Apa1 (rs7975232) and Taq1 (rs731236), have been identified as risk factors for AD.51,52 Another polymorphism, CDX-2 (rs11568820), located in the binding site for the VDR transcription factor, is also associated with AD.53 In vitro studies have demonstrated that VDR overexpression reduces amyloid precursor protein in neuroblastoma cells.53
In a review of VitD receptor polymorphisms, striking differences were found in the frequency of the minor A allele among African (74%), Asian (43%), and white (19%) individuals,54 but there is limited information on the functional impact of these polymorphisms. A recent comprehensive analysis of factors that influence VitD levels among white individuals living in France55 found 2 polymorphisms in GC (which codes for the VitD-binding protein) that were independently associated with low plasma 25-OHD concentration. Although perhaps important for understanding genetic influences on VitD concentrations, this study did not include analyses of nonwhite individuals, thereby limiting inferences for our own findings.
There are a number of study limitations. First, individual blood samples were obtained at the time of enrollment and therefore varied widely by time of day and season. While group mean 25-OHD concentrations did not vary by season of draw, multiple factors may have influenced the average differences, thereby masking subtle underlying factors that could influence the results. Additionally, we did not measure dietary dairy intake, sun exposure, or exercise, each of which influences VitD levels.2,55 These limitations make assessment of potentially modifiable risk factors for VitD deficiency in our cohort more difficult. Moreover, genetic measures of polymorphism frequencies among candidate genes are not available at this time. Finally, although we sought to recruit a diverse cohort, the number of participants in this study was too small to examine interactions between race, degree of cognitive impairment, and VitD levels. We hypothesize that VitD affects cognition based on level and not race or ethnicity, but testing this hypothesis requires a much larger study population.
Conclusions
Our data support the common occurrence of VitD insufficiency among older individuals. In addition, these data show that African American and Hispanic individuals are more likely to have VitD insufficiency or deficiency. Independent of race or ethnicity, baseline cognitive ability, and a host of other risk factors, VitD insufficiency was associated with significantly faster declines in both episodic memory and executive function performance, which may correspond to elevated risk for incident AD dementia. Given that VitD insufficiency is medically correctable, well-designed clinical trials that emphasize enrollment of individuals of nonwhite race/ethnicity with hypovitaminosis D could be useful for testing the effect of VitD replacement on dementia prevention.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grant P30 AG010129 from the National Institutes of Health (Dr DeCarli).
Role of the Funder/Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Miller and DeCarli had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Miller, Harvey, Beckett, Green, Reed, Olichney, Mungas, DeCarli.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Miller, Harvey, DeCarli.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Harvey, Beckett.
Obtained funding: DeCarli.
Administrative, technical, or material support: Olichney, Mungas, DeCarli.
Study supervision: Olichney, DeCarli.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; Washington, DC: 2010. [Google Scholar]
- 2.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Eyles DW, Liu PY, Josh P, Cui X. Intracellular distribution of the vitamin D receptor in the brain: comparison with classic target tissues and redistribution with development. Neuroscience. 2014;268:1–9. doi: 10.1016/j.neuroscience.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 5.Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13(10):893–898. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 6.Annweiler C, Dursun E, Féron F, et al. “Vitamin D and cognition in older adults”: updated international recommendations. J Intern Med. 2015;277(1):45–57. doi: 10.1111/joim.12279. [DOI] [PubMed] [Google Scholar]
- 7.Littlejohns TJ, Henley WE, Lang IA, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer's disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205–1211. doi: 10.1093/gerona/gls107. [DOI] [PubMed] [Google Scholar]
- 9.Annweiler C, Fantino B, Schott AM, Krolak-Salmon P, Allali G, Beauchet O. Vitamin D insufficiency and mild cognitive impairment: cross-sectional association. Eur J Neurol. 2012;19(7):1023–1029. doi: 10.1111/j.1468-1331.2012.03675.x. [DOI] [PubMed] [Google Scholar]
- 10.Annweiler C, Montero-Odasso M, Llewellyn DJ, Richard-Devantoy S, Duque G, Beauchet O. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. J Alzheimers Dis. 2013;37(1):147–171. doi: 10.3233/JAD-130452. [DOI] [PubMed] [Google Scholar]
- 11.Llewellyn DJ, Lang IA, Langa KM, Melzer D. Vitamin D and cognitive impairment in the elderly US population. J Gerontol A Biol Sci Med Sci. 2011;66(1):59–65. doi: 10.1093/gerona/glq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64(12):1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 16.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5(9):735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 17.Hinton L, Carter K, Reed BR, et al. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis Assoc Disord. 2010;24(3):234–241. doi: 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 22.Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanics and non-Hispanic whites. J Int Neuropsychol Soc. 2005;11(5):620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mungas D, Beckett L, Harvey D, et al. Heterogeneity of cognitive trajectories in diverse older persons. Psychol Aging. 2010;25(3):606–619. doi: 10.1037/a0019502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mungas D, Reed BR, Marshall SC, González HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14(2):209–223. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- 25.Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16(4):347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- 26.Mungas D, Reed BR, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales: relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19(4):466–475. doi: 10.1037/0894-4105.19.4.466. [DOI] [PubMed] [Google Scholar]
- 27.Annweiler C, Schott AM, Allali G, et al. Association of vitamin D deficiency with cognitive impairment in older women: cross-sectional study. Neurology. 2010;74(1):27–32. doi: 10.1212/WNL.0b013e3181beecd3. [DOI] [PubMed] [Google Scholar]
- 28.Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138(6):353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 29.Elias MF, Elias PK, Robbins MA, Wolf PA, D'Agostino RB. Cardiovascular risk factors and cognitive functioning: an epidemiological perspective. In: Waldstein SR, Elias MF, editors. Neuropsychology of Cardiovascular Disease. Lawrence Erlbaum Associates; Mahwah, NJ: 2001. pp. 83–104. [Google Scholar]
- 30.Elias MF, Sullivan LM, D'Agostino RB, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35(2):404–409. doi: 10.1161/01.STR.0000103141.82869.77. [DOI] [PubMed] [Google Scholar]
- 31.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Early DR, Widaman KF, Harvey D, et al. Demographic predictors of cognitive change in ethnically diverse older persons. Psychol Aging. 2013;28(3):633–645. doi: 10.1037/a0031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Census Bureau [August 13, 2013];Census quickfacts. http://quickfacts.census.gov/qfd/states/00000.html.
- 34.US Administration on Aging [August 13, 2013];A statistical profile of Hispanic older Americans aged 65+ http://www.aoa.gov/Aging_Statistics/minority_aging/Facts-on-Hispanic-Elderly.aspx.
- 35.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51(2):169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 36.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64(4):570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 37.Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data, 1988-93. Cancer Causes Control. 1997;8(2):246–252. doi: 10.1023/a:1018432632528. [DOI] [PubMed] [Google Scholar]
- 38.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 39.Bouillon R, Carmeliet G, Daci E, Segaert S, Verstuyf A. Vitamin D metabolism and action. Osteoporos Int. 1998;8(suppl 2):S13–S19. doi: 10.1007/pl00022727. [DOI] [PubMed] [Google Scholar]
- 40.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30(2, pt 1):150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 41.Rostand SG. Vitamin D, blood pressure, and African Americans: toward a unifying hypothesis. Clin J Am Soc Nephrol. 2010;5(9):1697–1703. doi: 10.2215/CJN.02960410. [DOI] [PubMed] [Google Scholar]
- 42.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 44.Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimers Dement. 2014;10(3):296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- 45.Beydoun MA, Gary TL, Caballero BH, Lawrence RS, Cheskin LJ, Wang Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am J Clin Nutr. 2008;87(6):1914–1925. doi: 10.1093/ajcn/87.6.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Annweiler C, Annweiler T, Bartha R, Herrmann FR, Camicioli R, Beauchet O. Vitamin D and white matter abnormalities in older adults: a cross-sectional neuroimaging study. Eur J Neurol. 2014;21(12):1436–e95. doi: 10.1111/ene.12511. [DOI] [PubMed] [Google Scholar]
- 47.Hooshmand B, Lökk J, Solomon A, et al. Vitamin D in relation to cognitive impairment, cerebrospinal fluid biomarkers, and brain volumes. J Gerontol A Biol Sci Med Sci. 2014;69(9):1132–1138. doi: 10.1093/gerona/glu022. [DOI] [PubMed] [Google Scholar]
- 48.Annweiler C, Annweiler T, Montero-Odasso M, Bartha R, Beauchet O. Vitamin D and brain volumetric changes: systematic review and meta-analysis. Maturitas. 2014;78(1):30–39. doi: 10.1016/j.maturitas.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Andreasen N, Minthon L, Vanmechelen E, et al. Cerebrospinal fluid tau and Abeta42 as predictors of development of Alzheimer's disease in patients with mild cognitive impairment. Neurosci Lett. 1999;273(1):5–8. doi: 10.1016/s0304-3940(99)00617-5. [DOI] [PubMed] [Google Scholar]
- 50.Pericak-Vance MA, Bass MP, Yamaoka LH, et al. Complete genomic screen in late-onset familial Alzheimer disease: evidence for a new locus on chromosome 12. JAMA. 1997;278(15):1237–1241. [PubMed] [Google Scholar]
- 51.Gezen-Ak D, Dursun E, Ertan T, et al. Association between vitamin D receptor gene polymorphism and Alzheimer's disease. Tohoku J Exp Med. 2007;212(3):275–282. doi: 10.1620/tjem.212.275. [DOI] [PubMed] [Google Scholar]
- 52.Lehmann DJ, Refsum H, Warden DR, Medway C, Wilcock GK, Smith AD. The vitamin D receptor gene is associated with Alzheimer's disease. Neurosci Lett. 2011;504(2):79–82. doi: 10.1016/j.neulet.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Hara K, Van Baaren JM, et al. Vitamin D receptor and Alzheimer's disease: a genetic and functional study. Neurobiol Aging. 2012;33(8):1844.e1–1844.e9. doi: 10.1016/j.neurobiolaging.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 54.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Touvier M, Deschasaux M, Montourcy M, et al. Determinants of vitamin D status in Caucasian adults: influence of sun exposure, dietary intake, sociodemographic, lifestyle, anthropometric, and genetic factors. J Invest Dermatol. 2015;135(2):378–388. doi: 10.1038/jid.2014.400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.