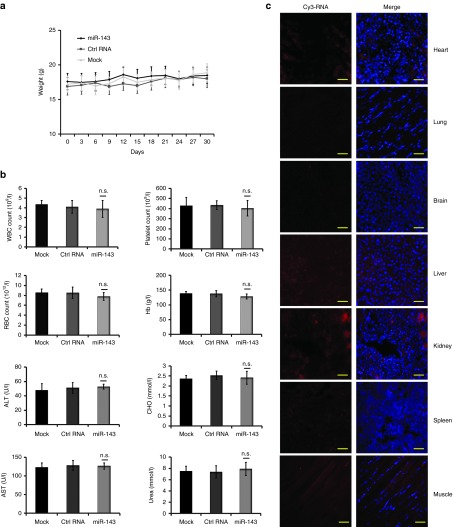
Figure 5.
Toxicity analyses following intravenous delivery of encapsulated miRNA agomir in triple-negative breast cancer (TNBC) xenografts. Another three randomized groups of TNBC tumor-bearing xenografts were established (n = 3 mice in each group). When tumor diameters reached ~5 mm, encapsulated miRNA agomirs were administered by tail vein injection at 1.5 mg/kg of body weight every 3 days for 10 cycles. (a) Body weights were measured every 3 days throughout the study. (b) Routine blood tests and biochemical tests were performed in normal BALB/c athymic nude mice after treatment with 1.5 mg/kg of body weight miR-143 agomir or Ctrl RNA agomir or phosphate buffered saline (PBS). (c) Cy3 signals were examined in heart, lung, brain, liver, kidney, spleen, muscle tissue slices shown in the left panels to evaluate the biodistribution of Cy3-labeled miR-143 agomir. The merged images with 4′,6-diamidino-2-phenylindole nucleus counterstained are shown in the right panels. Scale bars: 50 μm. Data are presented as mean ± SD.