Abstract
OBJECTIVE
We sought to examine the association between maternal serum 25-hydroxyvitamin D (25[OH]D) concentration in early pregnancy and the subsequent diagnosis of preeclampsia (PE).
STUDY DESIGN
This was a nested case-control study from 2 prospective Canadian cohorts conducted in Quebec City, Quebec, and Halifax, Nova Scotia, from 2002 through 2010. Participants were pregnant women (n=169 cases with PE and 1975 controls). Maternal serum was drawn <20 weeks of gestation, and 25(OH)D measurement was performed. Cases were ascertained from medical records. Logistic regression analysis was used to estimate adjusted odds ratios with 95% confidence intervals.
RESULTS
Women who developed PE had a significantly lower 25(OH)D concentration at a mean gestational age of 14 weeks compared with women in the control group (mean ± SD 25[OH]D 47.2 ± 17.7 vs 52.3 ± 17.2 nmol/L, P < .0001). Women with 25(OH)D <30 nmol/L compared to those with at least 50 nmol/L had a greater risk of developing PE (adjusted odds ratio, 2.23; 95% confidence interval, 1.29–3.83) after adjustment for prepregnancy body mass index, maternal age, smoking, parity, season and year of blood collection, gestational week at blood collection, and cohort site. Exploratory analysis with cubic splines demonstrated a dose-response relationship between maternal 25(OH)D and risk of PE, up to levels around 50 nmol/L, where the association appeared to plateau.
CONCLUSION
Maternal vitamin D deficiency early in pregnancy defined as 25(OH)D<30 nmol/L may be an independent risk factor for PE. The relevance of vitamin D supplementation for women of child-bearing age should be explored as a strategy for reducing PE and for promoting a healthier pregnancy.
Keywords: 25-hydroxyvitamin D, preeclampsia, vitamin D
One of the United Nations’ Millennium Development Goals for maternal health is to reduce maternal mortality by three-quarters by 2015.1 Hypertensive disorders of pregnancy, including preeclampsia (PE), are among the leading causes of maternal morbidity and mortality.2 The clinical manifestations of PE are well described as elevated blood pressure and protein-uria, 3 yet the etiology and prevention of the syndrome remain unclear.
Strategies to prevent PE include anti-platelet action of low-dose aspirin4 and dietary interventions with essential fatty acids,5 or vitamins C and E.6 While calcium supplementation is recommended by the World Health Organization 2 for regions where calcium intake is low, other calciotrophic factors such as vitamin D might be of higher relevance to industrialized nations.
Low vitamin D status is estimated to account for as much as 10% of pregnancy complications in Canada.7 Maternal vitamin D deficiency is common in northern nations, including Canada.8–10 According to national data, the average 25(OH)D concentration for Canadian women was 67 nmol/L and 41% took a supplement containing vitamin D.11 The Institute of Medicine (IOM)12 and Health Canada define vitamin D deficiency as serum 25- hydroxyvitamin D (25[OH]D) <30 nmol/L. The IOM recommends that 25(OH)D be >50 nmol/L for women of reproductive age as supported by a Recommended Dietary Allowance of 600 IU/d.12 However, oral intakes of vitamin D are sometimes unable to support physiological targets of 50 nmol/L of 25(OH)D in pregnancy, even with regular use of supplements.13 In addition, since vitamin D is a fat-soluble molecule, it may be sequestered in body fat, decreasing bioavailability and vitamin D status in obese women.14,15
Several epidemiologic studies have explored the association between vitamin D status and PE. In 2 of these studies, serum concentrations of 25(OH)D and 1,25-hydroxyvitamin D in early pregnancy were lower in women who subsequently developed PE.13,16 Overall, however, the epidemiological evidence is conflicting and previous studies are limited by small sample sizes or the absence of adjustment for relevant confounders, such as season, smoking, or parity.13,16–22 Moreover, there is a lack of consensus about the threshold of 25(OH)D at which the risk of PE is decreased. Given a strong biological rationale for a link between vitamin D metabolism and the physiopathology of PE through immunomodulation16 and angiogenesis,23 stronger epidemiologic evidence from studies with larger sample sizes and control for important confounding variables is needed.
Therefore, we conducted a large nested case-control study from 2 cohorts from Eastern Canada. The primary objective was to test whether vitamin D deficiency <20 weeks of gestation, defined as 25(OH)D <30 nmol/L, was associated with an increased risk of PE. The secondary objective was to explore the dose-response relationship between maternal serum 25(OH)D in early pregnancy and the risk of PE.
Materials and Methods
Cohort design, participant recruitment, and blood collection
This nested case-control study originated from separate Canadian cohort studies of pregnant women recruited in Halifax, Nova Scotia, and in Quebec City, Quebec. Blood samples were collected <20 weeks of gestation prior to diagnosis of PE.
In Halifax, Nova Scotia, pregnant women attending the blood collection services laboratory for routine prenatal blood screening were invited to participate <20 weeks of gestation, based on self-report and if they were planning on delivering at the Izaak Walton Killam Health Centre as previously described.24 Gestational age was confirmed using last menstrual period or ultrasound estimates. Blood samples were collected from October 2002 through July 2005. Recruitment into the Quebec City, Quebec, cohort spanned March 2005 through April 2010 at the first routine visit to the hospital between week 14–17 (2005 through 2008) or at the dating ultrasound between week 8–12 (2008 through 2010). In both cohorts, blood samples were sent to the clinical laboratories for procurement and then to the research laboratory for storage at −80°C.
Selection of cases and controls
The study was designed to examine several outcomes, and all cases of pregnancy loss, gestational diabetes, PE, preterm delivery, or having an infant who was low birthweight or small for gestational age were included. For the present analysis, cases were defined as women with PE, defined according to the Canadian Hypertension Society3 as gestational-onset hypertension with proteinuria (blood pressure >140/90 mm Hg after 20 weeks’ gestation with urinary protein >300 mg/d or urinary dipstick ≥2+).
Noncases were randomly selected from the women without any of the case outcomes with frequency matching to the combined case group on the following factors: study site (Halifax, Nova Scotia, or Quebec City, Quebec), gestational week of recruitment (<10, 10–13, 14–15, 16–17, 18–19), season (October through December, January through March, April through September), and year of blood sample collection, to ensure similar conditions at the time of blood collection between cases and controls. For the present analysis, controls were defined as the noncases plus participants randomly selected from those with the other case conditions at the frequency of these conditions within the entire cohort. Specifically, 219 subjects with a case condition (eg, gestational diabetes, low birthweight, preterm birth, or small for gestational age) were added to the control group (representing 11% of the controls). This was in contrast to 41% of the cases who had 1 of these other conditions. Women with preexisting hypertension were excluded.
After excluding withdrawals and participants with missing essential outcome information (no gestational age at enrollment), the total number of cohort participants delivering live births was 2036 in Halifax, Nova Scotia, and 7184 in Quebec City, Quebec. Women with multiple gestations were subsequently excluded. The final number of participants in both cohorts with available information on 25(OH)D concentration for the current analysis was 2144: 169 cases with PE and 1975 controls.
Questionnaires and medical chart reviews
At both sites, exposure and covariate information was surveyed including maternal age, education level, family income, prepregnancy weight, height, smoking habits, chronic medical conditions, pregnancy history, physical activity, and caffeine intake in early pregnancy. In Halifax, Nova Scotia, the questionnaire was completed during the 20th week of pregnancy and in Quebec City, Quebec, between 18–28 weeks. After delivery, medical charts were reviewed for all participants by trained personnel to collect data on prenatal ultrasound dating, maternal blood pressure readings and status, urinary protein findings, use of medications for hypertension before and during pregnancy, maternal weight at the time of delivery, antenatal hospital admissions, gestational age at delivery, and infant birthweight, sex, and outcome.
Measurement of vitamin D status
Serum samples were shipped on dry ice to McGill University and stored at −80°C until analysis of total 25(OH)D concentration using an automated chemiluminescence immunoassay (Liaison; DiaSorin, Stillwater, MN). Internal quality control measures included duplicate measure of the high and low kit controls and a pooled serum sample from non-pregnant healthy adults. The laboratory participated in the vitamin D external quality assessment scheme program and obtained a certificate of proficiency for 2011 through 2012 and 2012 through 2013, which reflects that ≥80% of the reported results fell within 30% of the all-laboratory trimmed mean. The laboratory also received a quality assurance certified value from the National Institute for Standards and Technology (NIST). Accuracy using NIST vitamin D controls indicated a 3.3% difference of the all-laboratory trimmed mean from the NIST reference measurement procedure in October 2012 and a 6.3% difference in January 2013. The interassay coefficient of variation was 5.7% and intraassay coefficient of variation ranged from 1.4–7.6% with a mean of 3.9% across runs. In addition, 25(OH)D analyzed in 40 samples with both the chemiluminescence immunoassay and the liquid chromatography tandem-mass spectroscopy method were compared yielding a bias of 3.0 ± 12.4 nmol/L.
Statistical analyses
Covariates assessed for confounding were selected based on factors noted in previous research and related to PE and/or vitamin D17,22,25–27: maternal age (<25, 25–<30, 30–<35, ≥35 years); maternal prepregnancy body mass index (BMI), categorized in 3 groups of underweight and normal weight (<24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2); marital status (married/common-law, single); maternal highest education qualification (≤high school, some or all college, technical or some or all university); annual family income (<$40,000 and ≥$40,000 Canadian); smoking (nonsmoker and/or ex-smoker who quit before pregnancy vs smoked at some time during pregnancy); parity (nulliparous vs primiparous/multiparous); and physical activity (number of times done for 20–30minutes in the first half of pregnancy) classified in 6 groups (none, 1 time/mo, 2–3 times/mo, 1 time/wk, 2–3 times/wk, ≥4 times/wk). Other potential confounders included infant sex (female or male); caffeine consumption in early pregnancy, calculated based on the daily number of cups of coffee or tea (0, 0–149, 150–299, and ≥300 mg/d); residence (rural or urban); season of blood collection (spring, summer, autumn, or winter); and year of blood collection (2002 through 2010). Ethnicity of parents, dairy product consumption, and prenatal supplements were only available for the Quebec City, Quebec, cohort and were, therefore, not included in the analyses.
Characteristics of mothers who developed PE and mothers who did not were compared using χ2 or Fisher exact test for categorical variables and Student t test for continuous variables. Logistic regression analyses adjusting for potential confounding factors were used to estimate the risk (adjusted odds ratios [aOR] with 95% confidence intervals [CI]) of PE according to the exposure variable, serum 25(OH)D concentrations <20 weeks of gestation. The cutoffs established by the IOM in 201112 were used to categorize 25(OH)D: <30, 30–49.9, and ≥50 nmol/L; with ≥50 nmol/L set as the referent category. Subgroup analysis based on 25(OH)D >75 nmol/L was not possible as only 9% of controls and 6% of cases were in this category. The following potential prespecified variables were forced in the model: maternal age, smoking, parity, prepregnancy BMI, season and year of blood collection, gestational age at blood collection, and study site (Halifax, Nova Scotia, or Quebec City, Quebec). Other covariates that were associated with PE with a P value<.2 in unadjusted analysis were assessed to determine whether they confounded the relationship between 25(OH)D and PE: if removing the covariate did not change the odds ratio (OR) for the association between 25(OH)D and PE by >5%, it was removed from the adjustment model. Effect modification of vitamin D status with prepregnancy BMI and with smoking was tested using the likelihood ratio test. Then, spline regressions were developed to assess the dose-response relationship between 25(OH)D concentration and PE risk. All analyses were performed with Statistical Analysis Software, Version 9.3 (SAS Institute, Cary, NC).
Ethical approval
The study was approved by the research ethics boards of the Izaak Walton Killam Health Centre in Halifax, Nova Scotia; the Center Hospitalier Universitaire de Québec; and McGill University in Montreal, Quebec. All participants signed informed written consent.
Results
Participants’ characteristics
Of the number of cohort participants (9220: 2036 from Halifax, Nova Scotia, and 7184 from Quebec City, Quebec), 169 (1.8%) developed PE. Table 1 presents the characteristics of the study population by case-control status. There were no significant differences between mothers who developed PE and mothers who did not develop PE in terms of age, marital status, education, family income, infant sex, smoking in pregnancy, caffeine consumption, physical activity, and living in an urban or rural area. There was a higher proportion of obesity, preexisting diabetes, and nulliparity in cases compared to controls. Likewise, while not a potential confounder, women with PE had a higher proportion of caesarean delivery at a lower mean gestational age.
TABLE 1.
Participant characteristics
| Characteristic | Control (n = 1975) | PEa (n = 169) | P valueb |
|---|---|---|---|
| GENERAL DEMOGRAPHICS | |||
| Maternal age, y | |||
| <25 | 240 (12) | 18 (11) | .63 |
| 25–<30 | 738 (37) | 70 (41) | |
| 30–<35 | 699 (35) | 60 (36) | |
| ≥35 | 298 (15) | 21 (12) | |
| Marital status | |||
| Married/common-law | 1685 (92) | 148 (94) | .37 |
| Single | 154 (8) | 10 (6) | |
| Highest maternal educational qualification | |||
| ≤High school | 393 (22) | 32 (21) | .94 |
| Some or all college, technical | 589 (33) | 51 (33) | |
| Some or all university | 807 (45) | 71 (46) | |
| Household annual income | |||
| <$40,000 | 378 (22) | 31 (21) | .56 |
| ≥$40,000 | 1309 (78) | 121 (79) | |
| Study site | |||
| Halifax, Nova Scotia | 490 (25) | 58 (34) | .0073 |
| Quebec City, Quebec | 1476 (75) | 111 (66) | |
| PREGNANCY VARIABLES | |||
| Prepregnancy body mass index, kg/m2 | |||
| Underweight and normal weight (<25) | 1257 (67) | 76 (47) | < .0001 |
| Overweight (25–29.9) | 377 (20) | 36 (22) | |
| Obese (≥30) | 254 (13) | 49 (30) | |
| Preexisting diabetes | |||
| No | 1957 (99.5) | 164 (97) | .0001 |
| Yes | 9 (0.5) | 5 (3) | |
| Parity | |||
| Primiparous/multiparous | 1053 (53) | 44 (26) | < .0001 |
| Nulliparous | 921 (47) | 125 (74) | |
| Sex of baby | |||
| Female | 958 (49) | 77 (46) | .43 |
| Male | 1007 (51) | 92 (54) | |
| Delivery | |||
| Vaginal | 1505 (77) | 103 (61) | < .0001 |
| Cesarean | 459 (23) | 66 (39) | |
| Gestational age at delivery, wk | |||
| Mean ± SD | 39.1 ± 1.5 | 37.3 ± 2.7 | < .0001 |
| LIFESTYLE VARIABLES | |||
| Smoking status | |||
| Nonsmoker or ex-smoker, quit before pregnancy | 1495 (81) | 133 (83) | .60 |
| Smoker: smoked at some time during pregnancy | 353 (19) | 28 (17) | |
| Caffeine from coffee or tea, mg/d | |||
| 0 | 656 (37) | 72 (47) | .11 |
| 1–149 | 651 (37) | 47 (31) | |
| 150–299 | 400 (23) | 29 (19) | |
| ≥300 | 67 (4) | 6 (4) | |
| Physical activity during pregnancy (20–30 min in free time) | |||
| None | 351 (20) | 42 (27) | .29 |
| 1 time/mo | 135 (8) | 13 (8) | |
| 2–3 times/mo | 205 (11) | 19 (12) | |
| 1 time/wk | 421 (24) | 31 (20) | |
| 2–3 times/wk | 479 (27) | 36 (23) | |
| ≥4 times/wk | 197 (11) | 14 (9) | |
| Residence | |||
| Urban | 1554 (87) | 138 (89) | .50 |
| Rural | 229 (13) | 17 (11) | |
Values are no. (%) or mean ± SD.
PE, preeclampsia.
Cases are defined as women with PE, diagnosed by blood pressure >140/90 mm Hg after 20 wks with proteinuria, and without preexisting hypertension;
P < .05 comparing mothers with PE and mothers with no PE, using t test for continuous variables and χ2 tests for differences for categorical variables.
Blood collection information
As shown in Table 2, at a mean gestational age of 14 weeks, the mean maternal serum 25(OH)D concentration was significantly lower in the group of women who later developed PE than in the control group (47.2 ± 17.7 vs 52.3 ± 17.2, P <.0001) and a higher proportion had 25(OH)D <30 nmol/L.
TABLE 2.
Blood collection information
| Characteristic | Controls (n = 1975) | PE (n = 169) | P valuea |
|---|---|---|---|
| Serum 25(OH)D concentration, nmol/L | |||
| Mean ± SD | 52.3 ± 17.2 | 47.2 ± 17.7 | .0002 |
| Median (IQR) | 51.9 (39.9–62.3) | 46.5 (35.5–57.5) | |
| Proportion <30 | 169 (9) | 27 (16) | .0001 |
| 30–49.9 | 740 (37) | 76 (45) | |
| ≥50 | 1066 (54) | 66 (39) | |
| Gestational age at blood collection, wk | |||
| Mean ± SD | 13.6 ± 3.0 | 13.8 ± 3.0 | .41 |
| Season | |||
| Spring | 479 (24) | 38 (22) | .58 |
| Summer | 384 (19) | 39 (23) | |
| Autumn | 567 (29) | 43 (25) | |
| Winter | 545 (27) | 49 (29) | |
| Year | |||
| 2002 through 2005 | 669 (34) | 71 (42) | .03 |
| 2006 through 2010 | 1306 (66) | 98 (58) | |
Values are no. (%) unless otherwise specified.
IQR, interquartile range; PE, preeclampsia; 25(OH)D, 25-hydroxyvitamin D.
P<.05 comparing mothers with PE and mothers with no PE, using t test for continuous variables and χ2 tests for differences for categorical variables.
Logistic regression analyses
In the unadjusted model (Table 3), maternal vitamin D deficiency, defined by maternal 25(OH)D <30 nmol/L, was associated with an increased odds of PE (OR, 2.48; 95% CI, 1.51–4.08) compared with 25(OH)D concentrations of at least 50 nmol/L. After adjusting for covariates, a similarly increased odds of PE was observed (aOR, 2.23; 95% CI, 1.29–3.83) among women with vitamin D deficiency, compared with women with 25(OH)D of at least 50 nmol/L. When comparing 25(OH)D concentrations of 30–49.9 nmol/L to at least 50 nmol/L, there was a modest increased risk of PE after adjusting for covariates.
TABLE 3.
Unadjusted and adjusted odds ratios for preeclampsia according to vitamin D status in early pregnancy
| 25(OH)D, nmol/L | Controls | PE | Unadjusted OR (95% CI) | Adjustedb OR (95% CI) | ||
|---|---|---|---|---|---|---|
| na | % | na | % | |||
| <30 | 153 | 8.1 | 24 | 14.9 | 2.48 (1.51–4.08) | 2.23 (1.29–3.83) |
| 30–<50 | 707 | 37.5 | 72 | 44.7 | 1.61 (1.14–2.28) | 1.51 (1.04–2.19) |
| ≥50 | 1027 | 54.4 | 65 | 40.4 | Reference | Reference |
| Total | 1887 | 161 | ||||
CI, confidence interval; OR, odds ratio; PE, preeclampsia; 25(OH)D, 25-hydroxyvitamin D.
n Is same in unadjusted model as in adjusted model–women with missing values for any of variables in adjusted model are excluded;
Adjusted for prepregnancy body mass index, parity, maternal age, smoking, season of blood collection, year of blood collection, gestational age at blood collection, and study site (Halifax, Nova Scotia, and Quebec City, Quebec).
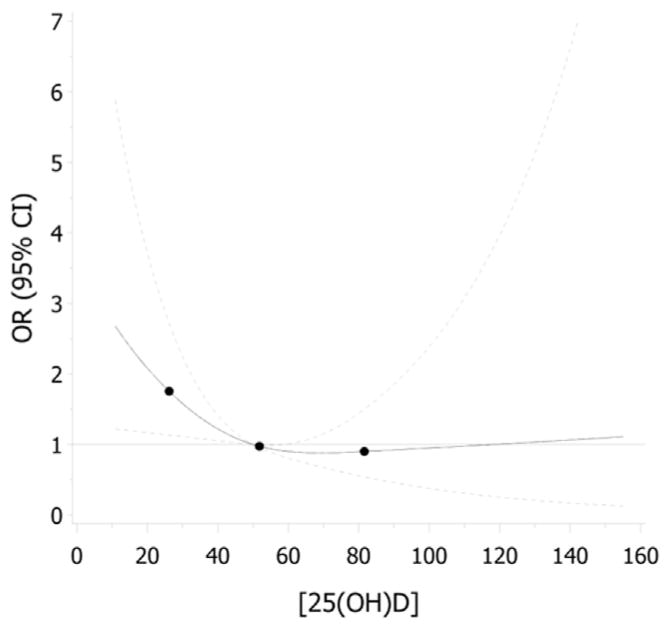
Dose-response association
The Figure shows the dose-response association between maternal serum 25(OH)D concentration at <20 weeks’ gestation and the risk of PE. Exploratory analysis with cubic splines demonstrated a dose-response relationship between maternal 25(OH)D and risk of PE, up to 50 nmol/L, where the association appeared to plateau. The very wide CI >75 nmol/L reflects the small number of participants with values in this range. Because of the limited precision in the risk estimates for values >75 nmol/L, it is not possible to make inferences for women with these higher values.
FIGURE. Dose-response association between maternal serum 25(OH)D concentrations at <20 weeks’ gestation and risk of preeclampsia.
Solid line represents the odds ratio (OR) relative to 50 nmol/L, and dotted lines represent 95% confidence intervals (CIs). Dose-response relationship was modeled with restricted cubic splines using logistic regression. Adjustment is made for prepregnancy body mass index, parity, and variables forced in model maternal age, smoking during pregnancy, season of blood collection, year of blood collection, gestational age at blood collection, and study site (Halifax, Nova Scotia, or Quebec City, Quebec).
25(OH)D, 25-hydroxyvitamin D.
Comment
This nested case-control study aimed to determine the association between low vitamin D status in early pregnancy and the subsequent diagnosis of PE. Maternal vitamin D deficiency, defined as 25(OH)D <30 nmol/L, was associated with a doubled odds of PE compared to concentrations >50 nmol/L. This relationship was significant both before and after adjustment for factors known to be related to PE and/or vitamin D status including maternal age, nulliparity,28 season,10,25 and prepregnancy BMI.14,15,27
To our knowledge, the present investigation is the largest case-control study in Canada on the association between 25(OH)D and PE. We extended earlier work by Bodnar et al13 and Baker et al16 in the sense that a low vitamin D status in early pregnancy was associated with an increased risk of PE. Similarly, 25(OH)D concentration at the time of diagnosis of early-onset severe PE was significantly lower among patients compared to healthy controls (44.9 vs 79.9 nmol/L; P < .001).29 In these studies ≤55 cases were studied, thus our study improves the precision of the ORs while accounting for multiple covariates.
One of the main determinants of vitamin D status is ultraviolet B exposure. 10,25,30,31 Previous studies have either not accounted for season13 or were predominantly conducted in the warmer months.16 Blood samples were equally collected across the seasons in our study. In addition, the definition of vitamin D deficiency has changed over time.12,32 For example, Bodnar et al13 used the 1997 IOM definition of 25(OH)D <37.5 nmol/L,32 and observed a 5-fold increase in the odds of PE (aOR, 5.0; 95% CI, 1.7–14.1). Baker et al16 used more contemporary cut-off points: 50 nmol/L and 75 nmol/L.12,30,31 They found a 5-fold increase in the odds of PE when comparing 25(OH)D <50 nmol/L with concentrations ≥75 nmol/L (aOR, 5.4; 95% CI, 2.0–14.5). In the present study, the 2011 IOM cutoffs of 30 and 50 nmol/L of 25(OH)D were used.12 We did not set study groups based on 25(OH)D >75 nmol/L because too few participants (9% of controls, 6% of cases) exceeded this concentration. Thus, future studies with higher maternal 25(OH)D concentrations are required to assess the benefits of higher vitamin D status.
Vitamin D supplementation in pregnancy helps to increase maternal concentrations of 25(OH)D,33,34 but there is limited evidence on the dosages that decrease the risk of PE and to date no randomized controlled trials (RCTs) have been published on vitamin D supplementation alone and risk of PE.26 Marya et al35 conducted a RCT at 20–24 weeks of gestation, with 200 pregnant women receiving calcium (375 mg/d) and vitamin D (1200 IU/d) supplements, and 200 controls. Although the supplemented group experienced a reduction in systolic and diastolic blood pressure, the reduction in the incidence of PE was not significant in the treated group compared with the untreated group (6% vs 9%). In addition, pregestational BMI, skin pigmentation, and blinding were not reported.33 In a very large prospective cohort study from Norway (n = 23,423), Haugen et al18 aimed to assess the association between vitamin D exogenous sources and PE. They measured supplementary intake of vitamin D in pregnancy and found a 27% reduction in risk of PE (OR, 0.73; 95% CI, 0.58–0.92), for women taking 400–600 IU/d as compared with no supplement. In the present study, 25(OH)D >50 nmol/L was associated with a decreased risk of PE, which may correspond to 600 IU/d of vitamin D, as per the IOM recommendations.12
This study is, however, not without limitations. Firstly, it is not possible to infer conclusions on dosages because vitamin D status was measured based on values of 25(OH)D, as compared to vitamin D intake. However, the serum biomarker maternal 25(OH)D is a composite indicator of vitamin D status, which takes into consideration exogenous and endogenous sources of vitamin D. In addition, prepregnancy BMI was self-reported, based on maternal weight and height before pregnancy, which is known to be systematically under-reported. 36 Nonetheless, this study also has methodological strengths. Maternal serum samples were collected before the diagnosis of PE and the assay used for 25(OH)D is accurate and precise.37 The large sample size and number of potential confounders that were assessed was also a major strength.
In summary, vitamin D deficiency <20 weeks of gestation, defined as 25(OH)D <30 nmol/L, was an independent risk factor of PE in our study, even after adjustment for covariates such as prepregnancy BMI. Based on the present study, reducing the proportion of women of childbearing age with vitamin D deficiency is important and may contribute to the prevention of adverse maternal health outcomes like PE. However, a causal relationship between vitamin D deficiency and PE cannot be inferred because of the observational design of this study. Well-designed RCTs will be necessary to establish the vitamin D intake from food and supplements required to reduce PE. Experimental and human studies are needed to clarify the physiological mechanisms underlying the association between vitamin D status and PE.
Acknowledgments
The authors acknowledge the Canadian Institute of Health Research for funding the study.
Footnotes
The authors report no conflict of interest.
Presented as a poster at the 10th Interdisciplinary Graduate Student (M. Achkar) Research Symposium, McGill University, Montreal, Quebec, Canada, April 1–2, 2014; in oral format at the First Annual Canadian National Perinatal Research Meeting, Banff, Alberta, Canada, Feb. 12–15, 2014; and in poster format at the Réseau de Recherche en Santé Buccodentaire et Osseuse retreat, Magog, Quebec, Canada, Jan. 16–17, 2014.
References
- 1.Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO recommendations for prevention and treatment of preeclampsia and eclampsia. Geneva: World Health Organization; 2011. [Google Scholar]
- 3.Helewa ME, Burrows RF, Smith J, Williams K, Brain P, Rabkin SW. Report of the Canadian Hypertension Society Consensus Conference, 1: definitions, evaluation and classification of hypertensive disorders in pregnancy. CMAJ. 1997;157:715–25. [PMC free article] [PubMed] [Google Scholar]
- 4.Benigni A, Gregorini G, Frusca T, et al. Effect of low-dose aspirin on fetal and maternal generation of thromboxane by platelets in women at risk for pregnancy-induced hypertension. N Engl J Med. 1989;321:357–62. doi: 10.1056/NEJM198908103210604. [DOI] [PubMed] [Google Scholar]
- 5.Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database Sys Rev. 2006;3:CD003402. doi: 10.1002/14651858.CD003402.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Rumbold AR, Crowther CA, Haslam RR, et al. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 7.Grant WB, Schwalfenberg GK, Genuis SJ, et al. An estimate of the economic burden and premature deaths due to vitamin D deficiency in Canada. Mol Nutr Food Res. 2010;54:1172–81. doi: 10.1002/mnfr.200900420. [DOI] [PubMed] [Google Scholar]
- 8.Canadian Pediatric Society. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12:583–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Meer IM, Karamali NS, Boeke AJ, et al. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr. 2006;84:350–3. doi: 10.1093/ajcn/84.1.350. quiz 468–9. [DOI] [PubMed] [Google Scholar]
- 10.Weiler HA, Leslie WD, Krahn J, et al. Canadian Aboriginal women have a higher prevalence of vitamin D deficiency than non-Aboriginal women despite similar dietary vitamin D intakes. J Nutr. 2007;137:461–5. doi: 10.1093/jn/137.2.461. [DOI] [PubMed] [Google Scholar]
- 11.Janz T, Pearson C. Vitamin D blood levels of Canadians. Statistics Canada; [Accessed Oct. 12, 2013]. Catalogue no. 82–624-X. Available at: http://www.statcan.gc.ca/pub/82-624-x/2013001/article/11727-eng.htm. [Google Scholar]
- 12.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine (US) Committee to Review Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 13.Bodnar LM, Catov JM, Simhan HN, et al. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–22. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodnar LM, Catov JM, Roberts JM, et al. Prepregnancy obesity predicts poor vitamin D status in mothers and their neonates. J Nutr. 2007;137:2437–42. doi: 10.1093/jn/137.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 16.Baker AM, Haeri S, Camargo CA, et al. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95:5105–9. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodnar LM, Simhan HN, Catov JM, et al. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology. 2014;25:207–14. doi: 10.1097/EDE.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haugen M, Brantsaeter AL, Trogstad L, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20:720–6. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 19.Powe CE, Seely EW, Rana S, et al. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010;56:758–63. doi: 10.1161/HYPERTENSIONAHA.110.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shand AW, Nassar N, Von Dadelszen P, et al. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG. 2010;117:1593–8. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 21.Wei SQ, Audibert F, Hidiroglou N, et al. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG. 2012;119:832–9. doi: 10.1111/j.1471-0528.2012.03307.x. [DOI] [PubMed] [Google Scholar]
- 22.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26:889–99. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 23.Grundmann M, Haidar M, Placzko S, et al. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Cell Physiol. 2012;303:C954–62. doi: 10.1152/ajpcell.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodds L, Fell DB, Dooley KC, et al. Effect of homocysteine concentration in early pregnancy on gestational hypertensive disorders and other pregnancy outcomes. Clin Chem. 2008;54:326–34. doi: 10.1373/clinchem.2007.097469. [DOI] [PubMed] [Google Scholar]
- 25.Sloka S, Stokes J, Randell E, et al. Seasonal variation of maternal serum vitamin D in Newfoundland and Labrador. J Obstet Gynaecol Can. 2009;31:313–21. doi: 10.1016/S1701-2163(16)34148-2. [DOI] [PubMed] [Google Scholar]
- 26.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26(Suppl):75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh SW. Obesity: a risk factor for preeclampsia. Trends Endocrinol Metab. 2007;18:365–70. doi: 10.1016/j.tem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Conde-Agudelo A, Belizan JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG. 2000;107:75–83. doi: 10.1111/j.1471-0528.2000.tb11582.x. [DOI] [PubMed] [Google Scholar]
- 29.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203:366e1–6. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academies Press; 1997. pp. 250–87. [PubMed] [Google Scholar]
- 33.De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;2:CD008873. doi: 10.1002/14651858.CD008873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxemia of pregnancy. Gynecol Obstet Invest. 1987;24:38–42. doi: 10.1159/000298772. [DOI] [PubMed] [Google Scholar]
- 36.Elgar FJ, Stewart JM. Validity of self-report screening for overweight and obesity: evidence from the Canadian community health survey. Can J Public Health. 2008;99:423–7. doi: 10.1007/BF03405254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42:1549–56. doi: 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]