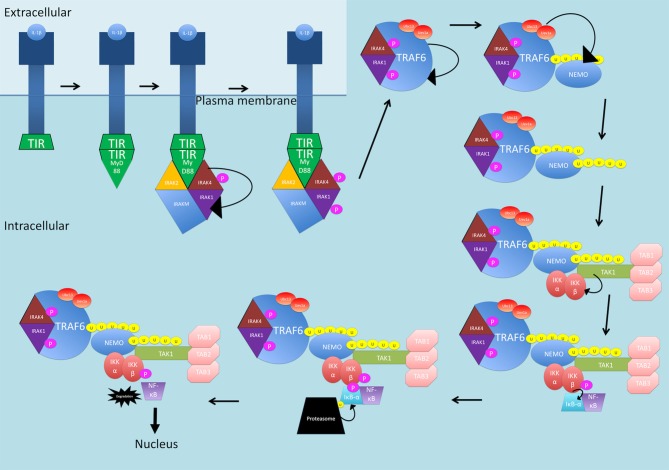
Figure 2.
The IL-1β-activated canonical NF-κB signaling pathway. The receptor is activated by IL-1β and recruits MyD88 through homophilic interactions between toll/interleukin-1 receptor (TIR) domains. MyD88 interacts with IRAK1, IRAK2, IRAKM and IRAK4. IRAK4 is phosphorylated and then phosphorylates and activates IRAK1. IRAK1 and IRAK4 interact with TRAF6 which self-ubiquitinates and also ubiquitinates NEMO through Ubc13-Uev1a, in complex with TRAF6. NEMO and TRAF6 recruit TAK1 in complex with TABs1–3. TAK1, activates IKKβ, which then phosphorylates IκB-α. IκB-α is then ubiquitinated and degraded, releasing NF-κB and allowing translocation into the nucleus. P, phosphate; U, ubiquitin.