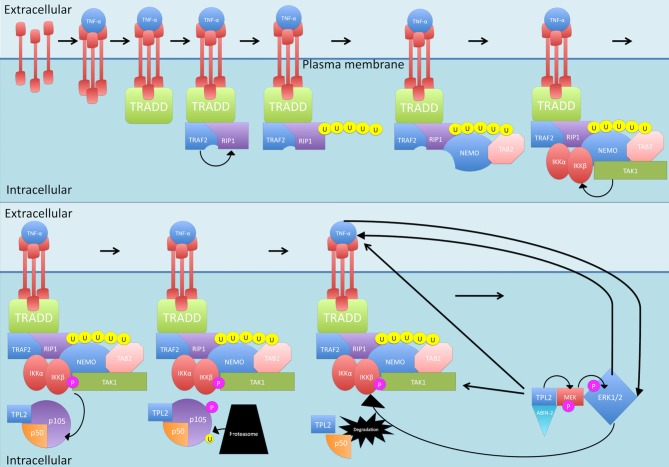
Figure 4.
Canonical activation of p50. Once activated by normal canonical signaling, IKKβ phosphorylates NF-κB subunit p105, which is the precursor of NF-κB subunit p50. Phosphorylation of p105 generates binding sites for ubiquitin ligases that then target p105 for degradation. p105 also inhibits tumor progression locus 2 (TPL2) by binding with it. Once p105 is degraded, TPL2 is released and stabilized by binding to MEK and ABIN-2. TPL2 then phosphorylates MEK which phosphorylates ERK, ERK1/2 leads to increased TNF-α production and can activate IKK. P, phosphate; U, ubiquitin.