Figure 8. The site and mechanism of action of O2 in cremaster muscle .
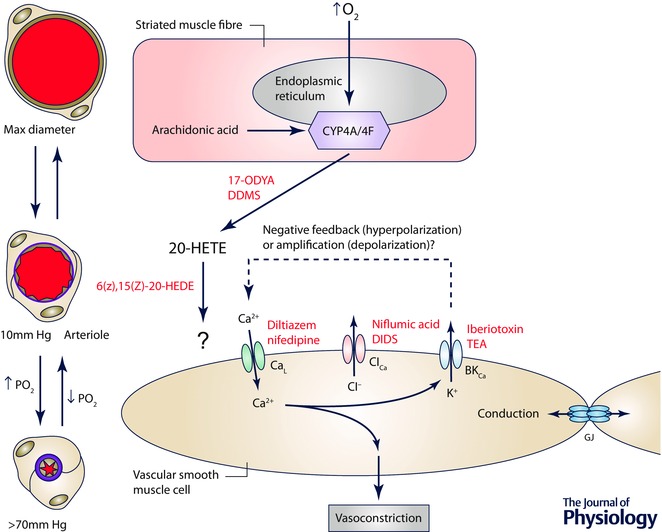
Schematic diagram of a striated muscle fibre (the proposed sensor cell in this tissue) and a smooth muscle cell replete with ion channels that may be involved in arteriolar O2 reactivity in this tissue. Elevated is sensed by cytochrome P4504A/4F ω‐hydroxylase (CYP4A/4F) located in the endoplasmic reticulum of striated muscle fibres, resulting in conversion of arachidonic acid into 20‐HETE, a process that can be inhibited by 17‐ODYA or DDMS. 20‐HETE then acts on smooth muscle cells to induce Ca2+ influx through L‐type Ca2+ channels (CaL), an increase in intracellular Ca2+ and vasoconstriction. As indicated by the ‘?’ next to the arrow connecting 20‐HETE and the smooth muscle cell, the precise receptor for 20‐HETE that is responsible for O2 reactivity is unclear because 20‐HETE has been proposed to close large conductance, Ca2+‐activated K+ channels (BKCa), which would lead to membrane depolarization activating CaL. In contrast, other studies suggest that BKCa serve a negative feedback role as they do in the cheek pouch. As in the cheek pouch it is proposed that O2‐induced depolarization of smooth muscle cells can be conducted along the vessel wall through gap junctions (GJ), consistent with the observed conducted vasoconstriction that has been observed experimentally. 6(Z),15(Z)‐20‐HEDE, 20‐hydroxy‐6Z,15Z‐eicosadienoic acid.
