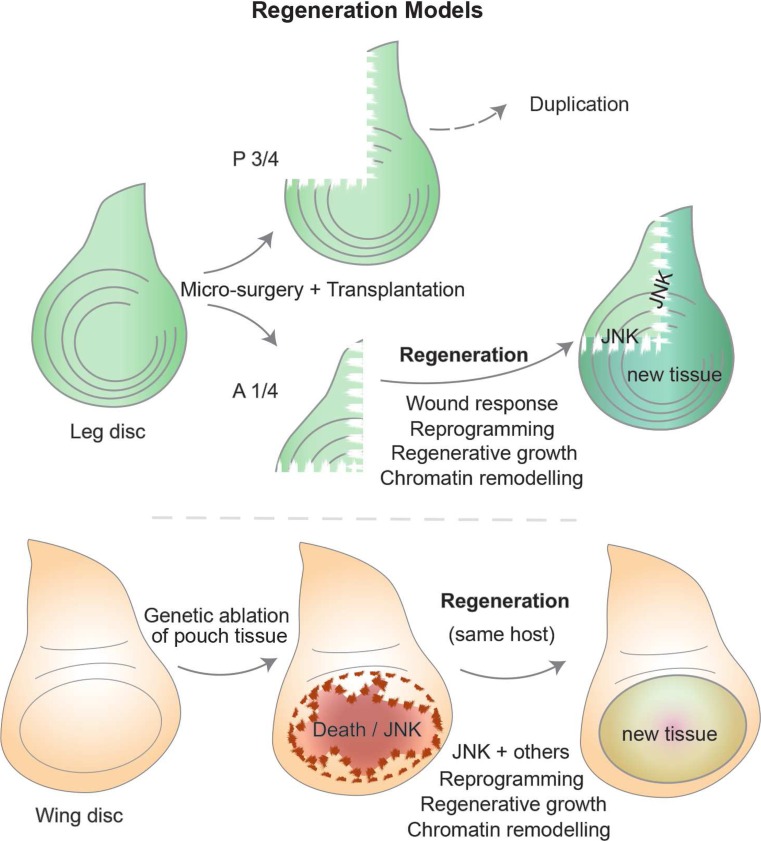
Fig. 5.
Regeneration in Drosophila imaginal discs. Imaginal discs are able to regenerate upon micro-surgery and culture through transplantation to host flies. The leg disc has routinely been used in fragmentation experiments (top), often with a standard cut that separates the “anterior one quarter” from the “posterior three quarters.” The larger fragment tends to result in tissue duplication, while the smaller fragment regenerates the remaining tissue with the correct pattern and function. The cut elicits a wounding-like response, followed by a phase of regenerative growth, for which chromatin remodeling is important during cellular reprogramming that can produce extra cells while maintaining the correct pattern and cellular identities. Recently developed tools (bottom) allow genetic-induced tissue ablation and regeneration within the same organism (overcoming the limitations of ablation and transplantation). Localized tissue ablation, for example, of the pouch region of the wing disc, can be spatially and temporally controlled. Different systems have been described (see main text), but generally, the design involves the following: a region-specific Gal4 is normally inhibited by ubiquitous Gal80 expression, which can be overcome by means of a temperature shift (with a temperature-sensitive form of Gal80 that is inactive at 29 °C or higher), which duration can be titrated to achieve specific conditions. During the period when Gal80 is inactive, Gal4 can activate a downstream UAS target that triggers apoptosis (e.g., using rpr, hid, egr, etc.). After an acute or controlled “damage-induction” phase, larvae are returned to a temperature that yields a functional Gal80 and thus inactivates the damage trigger, allowing tissues to regenerate during a “recovery” phase. Many of the hallmarks are conserved between the classical ablation experiments and the genetic-ablation systems, and the recent use of the latter promise considerable advantages that improve reproducibility and larger-scale experiments