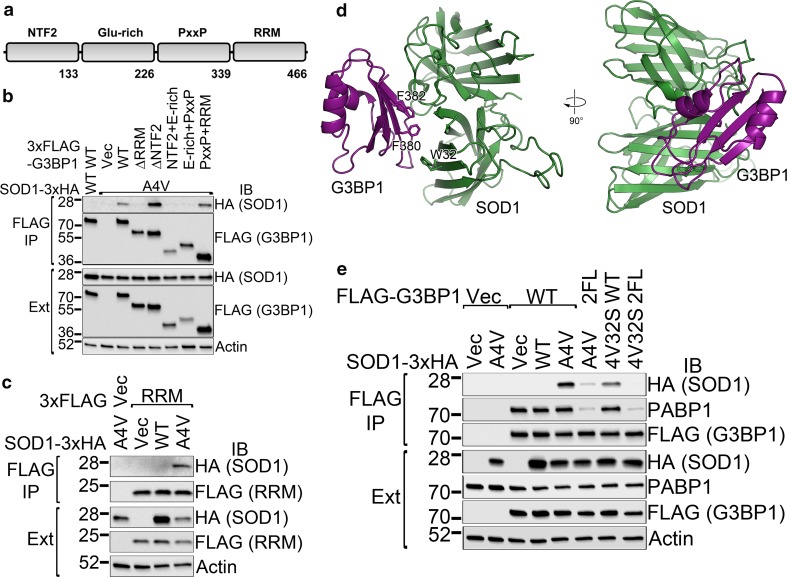
Fig. 4.
The binding of mutant SOD1 is mediated by the RRM domain of G3BP1. a The schematic domain structure of G3BP1. b The RRM domain of G3BP1 is necessary for the binding of mutant SOD1. A series of domain deletion mutants of G3BP1 were tested in FLAG-G3BP1 immunoprecipitations. c The isolated RRM domain of G3BP1 is sufficient for binding to mutant SOD1. FLAG immunoprecipitations were performed on cellular extracts with the expression of indicated constructs. d In silico docking of A4V mutant SOD1 (green) and the RRM domain of G3BP1 (purple). The F380 and F382 residues of G3BP1 and W32 of SOD1 are shown in stick representation. e The F380 and F382 residues of G3BP1 and the W32 residue of mutant SOD1 are important for the mutant SOD1–G3BP1 interaction. FLAG immunoprecipitations were performed followed by western blotting with the indicated antibodies. 2FL F380L/F382L double G3BP1 mutant, 4V32S A4V/W32S double SOD1 mutant, IB immunoblot, IP immunoprecipitation, Ext whole cell extracts