Abstract
Introduction:
OSTEOTRANS MX (Takiron Co, Ltd, Osaka, Japan) is a resorbable osteosynthetic material composed of an unsintered hydroxyapatite/poly-l-lactide composite, and its osteoconductive capacity has been documented. The authors here report their clinical experience using OSTEOTRANS MX.
Methods:
The authors treated 35 patients (19 men, 16 women; age, 14–88 years; mean ± standard deviation, 38.4 ± 19.9 years) with maxillofacial fractures. The authors used standard surgery to stabilize fractures in all patients, fitting resorbable plates (thickness, 1.0 or 1.4 mm) and screws (diameter, 2 mm) according to Arbeitsgemeinschaft für Osteosynthesefragen/Association (AO) for the Study of Internal Fixation guidelines.
Results:
All patients eventually achieved satisfactory healing with favorable restoration of form and function without foreign body reaction. Complications occurred in 3 patients—plate exposure in 2 and discomfort in 1. However, fracture sites healed in all patients. Scanning electron microscopy revealed that the devices bonded directly to the bone without interposition of nonmineralized tissue.
Conclusion:
OSTEOTRANS MX is a useful material with few complications. Its osteoconductive bioactivity is advantageous for the early functional improvement of maxillofacial fractures.
Keywords: Maxillofacial fractures, osteoconductive bioactivity, resorbable plate, unsintered hydroxyapatite/poly-l-lactide composite plate
Fixation of maxillofacial fractures with titanium miniplates is an established and widely used treatment. The mechanical properties of titanium, including its strength, ease of handling, lack of dimensional changes,1,2 minimal scatter on computed tomography (CT) scanning, and compatibility with radiography and magnetic resonance imaging,3 have prompted its widespread adoption as the general standard. However, fixation with titanium has several disadvantages, such as potential interference with facial growth,4,5 thermal sensitivity,3,6 plate migration,7,8 and interference with diagnostic imaging.9 The adverse effects of retained metallic devices also include osteopenia of cortical bone induced by protection from stress and corrosion.10 Thus, resorbable bone fixation devices have been developed.
OSTEOTRANS MX (Takiron Co, Ltd, Osaka, Japan), also called Super FIXSORB MX in Japan, is a bioactive, totally resorbable osteosynthetic bone fixation material. It is composed of a composite of fine particles of unsintered hydroxyapatite (u-HA) and poly-l-lactide (PLLA). The properties of this material have some clinical advantages, as follows: its mechanical strength closely approximates that of cortical bone, it has osteoconductive properties, and it makes direct contacts with the bone. Furthermore, because of its bioactive and biodegradable properties, the u-HA/PLLA composite has the potential for total replacement by bone.11 In this study, we report our clinical experience using OSTEOTRANS MX.
METHODS
Patients
We enrolled 35 patients (19 men, 16 women) aged 14 to 88 (mean ± standard deviation, 38.4 ± 19.9) years with maxillofacial fractures treated between 2009 and 2014 at the Division of Oral and Maxillofacial Surgery, Kagawa Prefectural Central Hospital, Kagawa, Japan. This study was approved by the Ethics Committee of Kagawa Prefectural Central Hospital (Approval No. 396). All patients provided written informed consent.
Methods
We used a standard process to stabilize fractures in all patients, fitting resorbable plates (thickness, 1.0 or 1.4 mm) and screws (diameter, 2 mm) (Fig. 1) according to Arbeitsgemeinschaft für Osteosynthesefragen/Association (AO) for the Study of Internal Fixation principles.
FIGURE 1.
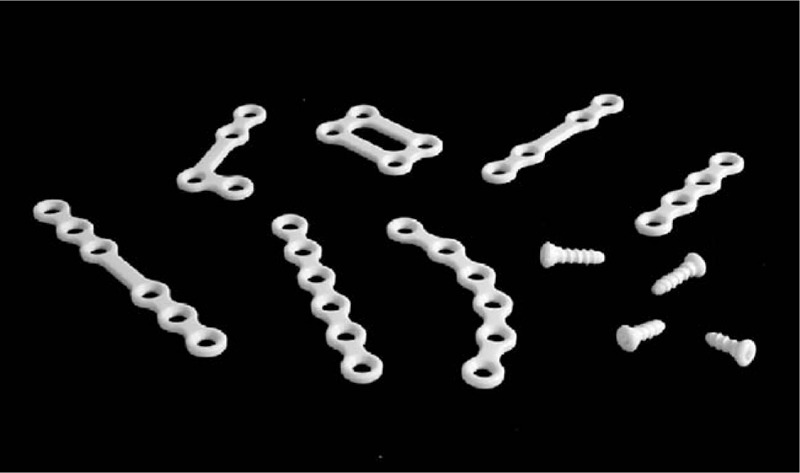
OSTEOTRANS MX (Takiron Co, Ltd, Osaka, Japan) composed of a forged unsintered hydroxyapatite/poly-l-lactide composite.
Le Fort I type fractures were treated with exposure of the anterior maxillary walls and piriform aperture. After the fractured segments were reduced, the maxillary segment was repositioned and fixed paranasally and at the infrazygomatic crest with 4-hole plates. Zygomaticomaxillary complex (ZMC) fractures were stabilized with 2-point fixation at the maxillary buttress and frontozygomatic suture following the ZMC reduction. A third fixation point at the infraorbital rim was used if the orbit was explored or reconstructed, and if this was performed, it helped to stabilize the fracture (Fig. 2).
FIGURE 2.
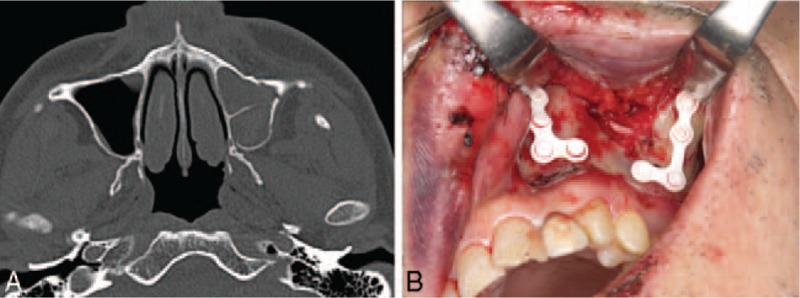
A 62-year-old male patient with a Le Fort I fracture from a traffic injury. (A) Preoperative computed tomography scan. (B) Transoral internal fixation.
Mandible fractures were manually reduced using reduction tools such as reduction forceps, and patients were placed into maxillomandibular fixation with the use of intermaxillary fixation screws with wire and heavy elastics. All fractures were treated with intraoral open reductions. Teeth in the line of fracture were extracted only if they demonstrated mobility, tooth root fracture, apical pathology, were nonrestorable, or interfered with the reduction of the fractures or occlusion. The internal fixation design was the 2-miniplate technique for the parasymphyseal, symphysis, and mandibular body fractures. At a minimum, 2 screws were placed on each side of the fracture (Fig. 3).
FIGURE 3.
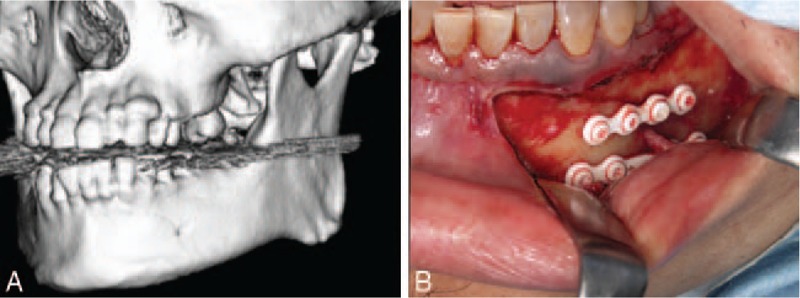
(A) A 52-year-old male patient with left mandibular body fracture from a fall injury. Preoperative computed tomography scan. (B) Transoral internal fixation.
After reducing the fracture, a template of the contours of the bone was created. The optimal shape and size of the plate to be used were selected and shaped according to this template. The material was warmed in hot water and adjusted by bending to match the contours of the template. Finally, the plate was affixed with screws. First, we drilled the bone to a depth of approximately 1 mm less than the total screw length. Second, we tapped the screw hole carefully with the screw tap to open it and rinsed out the bone chips. Third, the device was inserted and carefully fixed with screws to the bone. Regarding postoperative management, although we did not use intermaxillary fixation, guiding elastics were used to guide occlusion in mandibular fractures where appropriate.
Patients were followed up postoperatively, and the functional status and complications (including infection, swelling, nonunion, malunion, and exposure of the plate) of treatment were assessed through clinical and radiographical examinations.
The unexposed hardware side of the removed plates and screws and tissue adhered to the plate were subjected to evaluation as follows.
Scanning Electron Microscopy
A removed plate was fixed in 10% phosphate-buffered formalin to examine the attached tissues by scanning electron microscopy (SEM; S-3400N; Hitachi, Ltd, Tokyo, Japan).
Energy-Dispersive X-Ray Analysis
Elemental analyses were performed using an energy-dispersive X-ray analyzer (EX-350; Horiba, Ltd, Kyoto, Japan). The operating conditions used for analysis comprised an accelerating voltage of 20 kV and a working distance of 10 mm.
Measurement of Molecular Weight
The viscosity average molecular weight (Mv) of the polymer was determined from the intrinsic viscosity in chloroform at 25°C using the following equation12:
Measurement of Crystallinity
The crystallinity (%) of the polymer was determined using the following equation::
where ΔHTm is the melting enthalpy (J/g), and 93.7 is the theoretical melting enthalpy of 100% crystalline PLLA polymer (J/g).13
Histological Evaluation
We conducted a histological examination of tissue adhered to the plate that required removal from a patient experiencing discomfort. The tissue sample was fixed in 15% phosphate-buffered formalin, dehydrated with a graded 70% to 100% ethanol series, serially soaked in xylol, embedded in paraffin, and sectioned. The sections were stained with hematoxylin and eosin according to standard procedures.
RESULTS
Clinical Evaluation
All patients eventually achieved satisfactory healing with favorable restoration of form and function without foreign body reaction. The fracture lines healed completely in all patients (Table 1, Fig. 4). Complications occurred in 3 patients (3/35; 8.6%)—plate exposure (n = 2) and discomfort (n = 1). No nonunion or malunion occurred (Table 1). Plate exposure occurred in 2 patients with mandibular fractures (in the parasymphyseal region [n = 1] and in the body [n = 1]; Fig. 5). In both patients, plate exposure was recognized in the alveolar region and the exposed plate was removed after bone healing.
TABLE 1.
Clinical Characteristics of 35 Patients With Maxillofacial Fractures
| Patient | Age | Sex | Type of Fracture | Etiology | Outcome of Fracture Treatment | Postoperative Complication | Timing of Plate Removal (mo) | Observation Period (mo) |
| 1 | 56 | M | ZMC | Traffic accidents | Healing | Discomfort | 12 | |
| 2 | 34 | M | Mandibular body | Fall | Healing | Exposed plate | 8 | |
| 3 | 19 | M | Mandibular body | Traffic accidents | Healing | Exposed plate | 4 | |
| 4 | 24 | F | Symphysis | Fall | Healing | Non | 68 | |
| 5 | 18 | M | Le Fort I | Traffic accidents | Healing | Non | 63 | |
| 6 | 74 | F | ZMC | Traffic accidents | Healing | Non | 41 | |
| 7 | 42 | F | Le Fort I | Traffic accidents | Healing | Non | 30 | |
| 8 | 47 | F | Symphysis | Fall | Healing | Non | 21 | |
| 9 | 24 | F | Parasymphysis | Traffic accidents | Healing | Non | 20 | |
| 10 | 23 | M | Le Fort I | Traffic accidents | Healing | Non | 19 | |
| 11 | 30 | M | Le Fort I | Interpersonal conflicts | Healing | Non | 19 | |
| 12 | 75 | M | ZMC | Fall | Healing | Non | 19 | |
| 13 | 14 | F | Parasymphysis | Fall | Healing | Non | 17 | |
| 14 | 28 | F | Mandibular body | Traffic accidents | Healing | Non | 16 | |
| 15 | 48 | F | Le Fort I | Traffic accidents | Healing | Non | 16 | |
| 16 | 19 | F | Le Fort I | Fall | Healing | Non | 15 | |
| 17 | 78 | F | Mandibular body | Fall | Healing | Non | 15 | |
| 18 | 33 | F | Le Fort I/ II Fx | Traffic accidents | Healing | Non | 14 | |
| 19 | 29 | M | Le Fort I | Fall | Healing | Non | 13 | |
| 20 | 30 | F | ZMC | Interpersonal conflicts | Healing | Non | 12 | |
| 21 | 43 | M | Parasymphysis | Traffic accidents | Healing | Non | 12 | |
| 22 | 41 | M | Symphysis | Traffic accidents | Healing | Non | 11 | |
| 23 | 71 | M | Mandibular Condyle | Fall | Healing | Non | 11 | |
| 24 | 15 | F | Symphysis | Traffic accidents | Healing | Non | 10 | |
| 25 | 35 | M | Mandibular body | Fall | Healing | Non | 10 | |
| 26 | 26 | F | Symphysis | Fall | Healing | Non | 9 | |
| 27 | 19 | M | Symphysis | Sport | Healing | Non | 8 | |
| 28 | 38 | F | Parasymphysis | Fall | Healing | Non | 8 | |
| 29 | 17 | M | Mandibular condyle | Sport | Healing | Non | 8 | |
| 30 | 30 | F | Symphysis | Fall | Healing | Non | 8 | |
| 31 | 27 | M | Symphysis | Traffic accidents | Healing | Non | 7 | |
| 32 | 62 | M | Le Fort1 Fx | Traffic accidents | Healing | Non | 7 | |
| 33 | 88 | M | Parasymphysis | Fall | Healing | Non | 6 | |
| 34 | 52 | M | Parasymphyseal | Fall | Healing | Non | 6 | |
| 35 | 34 | M | Le Fort1 Fx | Fall | Healing | Non | 6 |
ZMC, zygomaticomaxillary complex.
FIGURE 4.
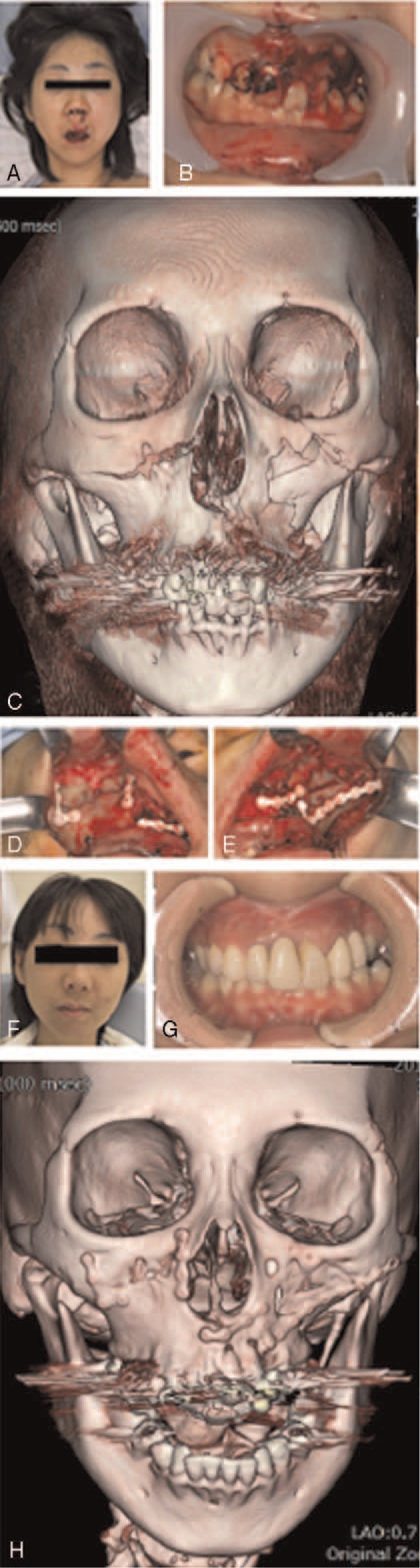
A 33-year-old female patient with a right Le Fort I and left Le Fort I/II fractures from traffic accident. (A) Frontal aspect at the emergency transportation, showing the remarkable facial deformation. (B) Intraoral view, showing his occlusal deviation due to midfacial fractures. (C) Three-dimensional CT showing complicated and dislocated right Le Fort I and left Le Fort I/ II fractures. (D, E) Bilateral transoral internal fixation with OSTEOTRANS MX. (F) Frontal aspect at 6-month follow-up, showing improvement of facial deformation. (G) Intraoral view, showing improvement of her occlusal. (H) Three-dimensional CT at postoperative 13-month, showing good bone integration. CT, computed tomography.
FIGURE 5.
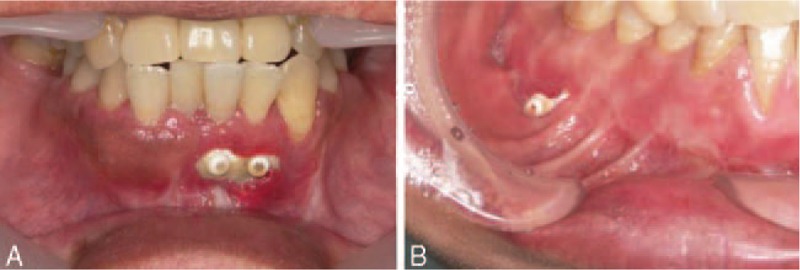
Plate exposure occurred in 2 patients with mandibular fractures. In both patients, exposure was recognized from the alveolar region and was not complete. (A) Plate exposure was recognized in the parasymphyseal region. (B) Plate exposure was recognized in the mandibular body.
One patient with a ZMC fracture reported discomfort (Fig. 6) of the infraorbital rim with no pus 10 months after surgery. The patient was monitored for a few months, but the situation did not improve, and at the patient's request, the plate was removed.
FIGURE 6.
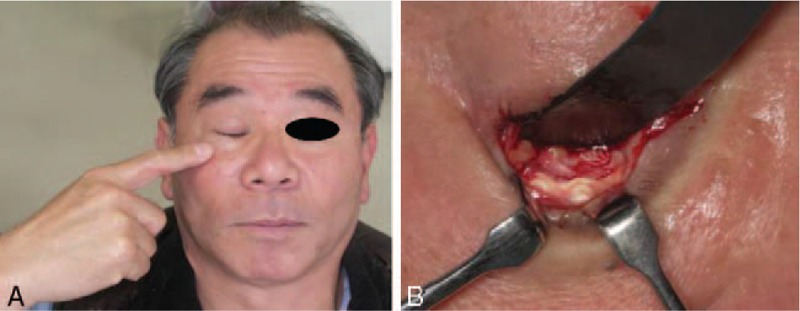
Discomfort occurred in 1 patient with a zygomaticomaxillary complex fracture. (A) The patient complained of discomfort of the infraorbital rim was evident 10 months after surgery. (B) The remnants of the plate and screws with fibrous tissue.
Scanning Electron Microscopy, Energy-Dispersive X-Ray Analysis, and Molecular Weight and Crystallinity Measurements
In a plate removed 4 months after surgery, the Mv and crystallinity of the material were 64 kDa and 54.1%, respectively. The Mv and crystallinity of the screw were 60 kDa and 54.4%, respectively (Table 2). On SEM, the outline of the plate remained distinct. Bone formation was seen in a circle around the plate, and remodeled trabecular bone had formed toward the center (Fig. 7A). The plate was observed to bond directly to the bone without interposition of nonmineralized tissue (Fig. 7B). On energy-dispersive X-ray analysis, the weight concentration of the deposits was 24.06 wt% calcium and 13.63 wt% phosphorus, similar to that of bone.14 Thus, we consider these deposits to be bone tissue.
TABLE 2.
Molecular Weight and Crystallinity Measurements of the Removed Plates
| Patient | Age | Sex | Timing of Plate Removal | Molecular Weight (kDa) | Crystallinity (%) |
| 1 | 19 | M | 4M | ||
| plate | 64 | 54.1 | |||
| screw | 60 | 53.4 | |||
| 2 | 34 | M | 8M | ||
| plate | 48 | 54.5 | |||
| screw | 39 | 64.8 | |||
| 3 | 56 | M | 12M | ||
| plate | 48 | 59 | |||
| screw | 26 | — |
FIGURE 7.
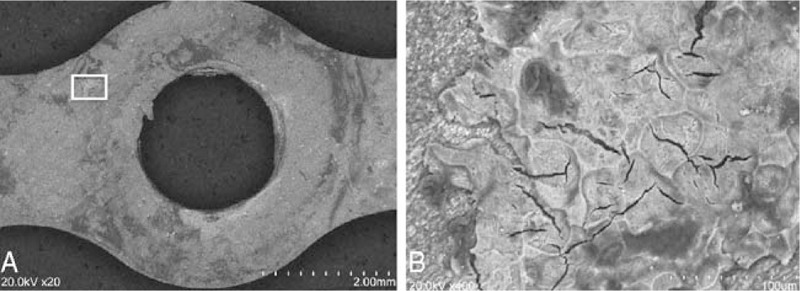
Scanning electron microscopy imaging of tissue attached to the plates. Bone formation in a circle around the plate and remodeled trabecular bone formation toward the center. The plate was observed to bond directly to the bone, with no interposition of nonmineralized tissue.
In a plate removed 8 months after surgery, the Mv and crystallinity of the plate were 48 kDa and 54.5%, respectively. The Mv and crystallinity of the screw were 39 kDa and 64.8%, respectively. Bone-like deposits were observed on SEM.
In a plate removed 1 year after surgery, the Mv and crystallinity of the plate were 48 kDa and 59%, respectively. The Mv of the screw was 26 kDa. The crystallinity of the screw could not be measured because the sample volume was too small. Bone-like deposits were observed on SEM. Fibrous tissue was also evident around the plate (Fig. 8).
FIGURE 8.
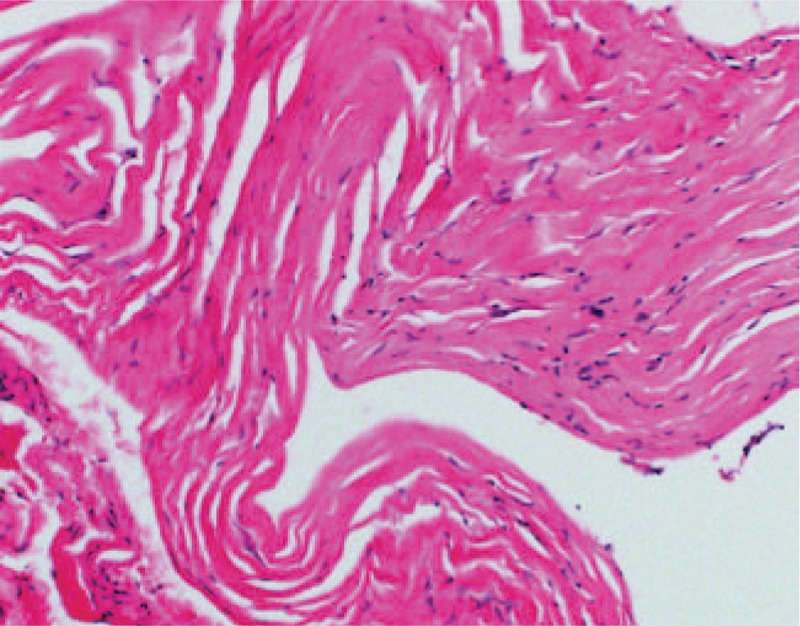
Histopathological examination confirmed the presence of fibrous tissue. The specimen was composed of dense fibrous tissue without infiltration of inflammatory cells or blood vessels, resembling the organizing phase of granulation tissue (magnification: ×200).
DISCUSSION
A resorbable osteosynthesis system has been developed for use similar to titanium mini plates in craniofacial, oral, maxillofacial, plastic, and reconstructive surgeries.2,15–19 Using this system, there is no need for a second surgery to remove the plate. OSTEOTRANS MX is made from a composite of uncalcined and unsintered u-HA and PLLA. Resorbable plates have been previously made from PLLA alone. However, PLLA osteosynthesis devices have several disadvantages, including lower dynamic strength, an inability to fuse with bone, and long resorption and replacement times.20–23 OSTEOTRANS MX was designed to overcome these problems, and the u-HA/PLLA composite material was developed by adding particulate resorbable uncalcined and unsintered u-HA to PLLA. Surgeons have recognized that bone fixation devices for use in maxillofacial surgery require much higher initial mechanical strength than natural cortical bone (which has a bending strength of 200 MPa). The u-HA/PLLA composite material has demonstrated higher mechanical strength, including bending strength, bending modulus, shears strength, and impact strength, than PLLA devices.24 Indeed, the material has an initial bending strength of 270 MPa,24 far higher than the bending strength of cortical bone. Hence, the high mechanical strength of OSTEOTRANS MX is thus advantageous for bone healing. Taken together, because of its bioactive, osteoconductive, as well as biodegradable properties, this u-HA/PLLA composite fixation system has the higher potentials for clinical advantages and may broaden the further indications in maxillofacial fracture repair surgery such as more complex, displaced, or comminuted fractures as a feasible next generative material.
The mechanical strength of the described osteosynthesis system is basically inferior to that of metallic, titanium devices. Recent evidence has revealed biomechanical differences between conventional bioresorbable plates and titanium hardware specifically designed for repair of mandible fractures. Stanton et al25 reported that the use of resorbable plating systems combined with a short postoperative period of intermaxillary fixation is a very effective method for internal fixation of mandibular fractures. Some studies demonstrated that conventional resorbable plates provide insufficient stability to counteract the masticatory forces of the mandible, resulting in higher rates of delayed union, as compared with a traditional titanium-based system.26,27 The resorbable plates used in these previous studies were constructed from PLLA alone or composites of PLLA and PLDLA/PGA. On the other hand, with respect to postoperative management, we did not use intermaxillary fixation, but rather elastics, to guide occlusion in mandibular fractures where appropriate. The intensity of this bioresorbable plate system is very high and its bending strength is far greater than that for cortical bone, whereas its modulus is almost equivalent to that of cortical bone.24 This plate system has high mechanical strength compared with that of other resorbable plates, although it is considered to be subject to appropriate occlusal management of maxillofacial fractures. One of the disadvantages of resorbable plates and screws is the lack of self-tapping. Because of these properties, it is necessary to pay much closer attention when using these devices for repair of complex midfacial injuries, severe displacement, and comminuted fractures. The thin and fragile maxillofacial bone regions, such as maxillary sinus and orbital walls, require careful attention when tapping in a technically demanding procedure.26–28 However, we occasionally selected a surgical procedure to perform only drilling without tapping, which was not a problem when carefully applied for fixation of extremely thin and fragile bone for repair of complex midfacial fractures.
We confirmed fracture healing postoperatively using CT scanning. We found that the fracture site had healed in all patients. It was possible to observe the plates because the u-HA particles render them radiopaque. CT scanning clearly showed the plates at various stages of fracture healing in our patients. We observed that the form of the plates was maintained until bone healing.
Shikinami et al29 reported that HA crystals were deposited and grew on the surface of this material 3 to 6 days after immersion in simulated body fluid, suggesting that it can bond directly to bone. Yasunaga et al30 reported that an u-HA/PLLA plate fixed to rabbit tibial cortices made direct contact with the bone with no intervening fibrous tissue. These reports indicate that u-HA/PLLA plates can bond directly to bone and possess osteoconductivity. We performed scanning capacitance microscopy analysis on an incompletely exposed plate after its removal 4 months postoperatively. Deposits of bone-like tissue were evident on the bone-contacting surface of the removed plate. The fibrous appearance of these deposits was suggestive of collagen fibrils of bone tissue, as evident in magnified images. This proved that the plates directly bonded to bone and proved the osteoconductivity of the u-HA/PLLA plate system. Its early osteoconductive bioactivity is advantageous for the early functional improvement of maxillofacial fractures.
Foreign-body reaction was not observed in all patients. The conventional devices made from the highly crystalline PLLA and PGA have shown long-term foreign body reactions.31,32 Furthermore, it has been confirmed that PGA degrades too rapidly after implantation and gives rise to considerable side effects that created inflammatory foreign-body reaction around the collapsing devices due to lowering of the pH and irritation from the fibrinous debris.33 Although the reinforced fabricated PLLA is more hydrophobic than PGA and therefore degrades slowly, PLLA-only devices have the potential to release uneven PLLA fragments at irregular time intervals during the degradation process. This can induce physical inflammatory responses.
On the other hand, uniform hydrolysis occurred throughout the PLLA matrix in the u-HA/PLLA material, and a steady release of small amounts of PLLA debris during PLLA degradation did not provoke adverse tissue responses.33 Additionally, a thin PLLA film provided interface with invading water molecules, which allowed homogeneous hydrolysis and steady degradation of PLLA. Thus, u-HA/PLLA material has the advantage of preventing a foreign-body reaction by its moderate and stable hydrolysis.34
Complications occurred in 3 patients, comprising exposed plates (n = 2) and discomfort (n = 1). The plate was removed in all these patients. Wound dehiscence and infection following plate exposure are the most frequent reasons for plate removal.35,36 Plate exposure occurred in 2 patients with mandibular fractures in the parasymphyseal region and the body. In both patients, plate exposure was recognized in the alveolar region and was incomplete. For increased strength, resorbable plates are of increased thickness; a resorbable plate must be thick to match the strength of a titanium plate.37 Although thicker plates are advantageous for mechanical strength, their increased mass makes them undesirable, as they are at a greater risk of exposure. The gingival alveolar portion is thin and easily suffers wound dehiscence, leading to exposure. Thus, it is necessary to cover it sufficiently using an oral vestibular approach.
Discomfort occurred in only 1 patient with a ZMC fracture. The patient complained of discomfort in the infraorbital rim approximately 1 year postoperatively. In this patient, we removed the plate after bone healing at the patient's request. The plate was palpable through the patient's facial skin, and the patient was experiencing a foreign-body sensation. In the immediate postoperative period, there was no discomfort, probably because of the general swelling of the face. However, the patient complained of mild discomfort after the facial swelling had subsided. Using thin plates is an option to reduce the discomfort caused by plates with midfacial fracture treatment, although there is controversy regarding their fixation strength.38,39 Yoshitaka et al40 reported that thicker plates were related to more discomfort than were thinner plates in their study. Polymerized PLLA plates that are used to fix unstable zygomatic fractures are voluminous; they are approximately 3 times the size of a titanium plate.41 In fact, these bioresorbable plates have a thickness of 1.0 or 1.4 mm. All of them are thicker than the thinnest titanium plate of 0.4 mm. Histopathological examination showed that the area of discomfort contained much fibrous tissue. Rarely, when the gross geometry of the implant is rapidly lost at approximately 1-year postsurgery, there is an increased risk of discomfort due to adverse tissue reactions.42 Covered with fibrous connective tissue around device, since there is no acute inflammation, it was not a foreign-body reaction due to the absorption process. In this patient, the removed plate had median Mv and low crystallinity compared with those of the new plate and screw. It was suggested that the stage of resorption was in progress, but it was not at the stage of phagocytic degradation by cells. Shikinami et al34 reported that the PLLA matrix in a composite was completely degraded and totally absorbed after 4 to 5 years, by which point natural new bone had replaced the majority of the u-HA particles. Additionally, it was previously reported that a decrease in Mv indicated the degradation of PLLA and the composite,22 and that the crystallinity of PLLA increased during hydrolysis.43 During the degradation of the screw and plate, the screw (which is in the bone) tends to decompose earlier than the plate (which is outside the bone). This indicates that degradation occurs more rapidly in the bone than in plates covered with fibrous tissue.
Therefore, when using a resorbable plate in an area of thin facial skin, such as the periorbital region or frontozygomatic suture, it is necessary to consider the following:
It is easier to palpate a thicker resorbable plate than a titanium plate.
It is possible that discomfort may be exacerbated over time because of a further increase in volume due to fibrous tissue covering the plate.
This material, composed of a reinforced composite of u-HA and PLLA, is suitable for use as an internal bone fixation device for facial fractures19 not only because of its mechanical properties but also because of its bioactivities, including osteoconduction.
We believe that this material may supersede titanium plates in the treatment of maxillofacial fractures if the appropriate technique is used to optimize the characteristics of this device
CONCLUSION
The present prospective clinical study of maxillofacial fractures showed successful treatment with resorbable implants composed of composite an unsintered hydroxyapatite/PLLA. All patients had successful fracture stabilization and reossification, with low complications. Its osteoconductive bioactivity is advantageous for the early functional improvement of maxillofacial fractures.
Acknowledgments
Some analyses, such as scanning electron microscopy, energy-dispersive X-ray analysis, and measurement of molecular weight, were done at Takiron Medical Institute, Kobe, Japan.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Bos RR. Treatment of pediatric facial fractures: the case for metallic fixation. J Oral Maxillofac Surg 2005; 63:382–384. [DOI] [PubMed] [Google Scholar]
- 2.Eppley BL, Sadove AM. Effects of resorbable fixation on craniofacial skeletal growth: modifications in plate size. J Craniofac Surg 1994; 5:110–114. [DOI] [PubMed] [Google Scholar]
- 3.Eppley BL, Sparks C, Herman E, et al. Effects of skeletal fixation on craniofacial imaging. J Craniofac Surg 1993; 4:67–73. [DOI] [PubMed] [Google Scholar]
- 4.Eppley BL, Platis JM, Sadove AM. Experimental effects of bone plating in infancy on craniomaxillofacial skeletal growth. Cleft Palate Craniofac J 1993; 30:164–169. [DOI] [PubMed] [Google Scholar]
- 5.Yaremchuk MJ, Fiala TGS, Barker F, Ragland R. The effects of rigid fixation on craniofacial growth of rhesus monkeys. Plast Reconstr Surg 1994; 93:1–10. [PubMed] [Google Scholar]
- 6.Manson PN, Hoopes JE, Su CT. Structural pillars of the facial skeleton: an approach to the management of Le Fort fractures. Plast Reconstr Surg 1980; 66:54–62. [DOI] [PubMed] [Google Scholar]
- 7.Fearon JA, Munro IR, Bruce DA. Observations on the use of rigid fixation for craniofacial deformities in infants and young children. Plast Reconstr Surg 1995; 95:634–637. [PubMed] [Google Scholar]
- 8.Yu JC, Bartlett SP, Goldberg DS, et al. An experimental study of the effects of craniofacial growth on the long-term positional stability of microfixation. J Craniofac Surg 1996; 7:64–68. [DOI] [PubMed] [Google Scholar]
- 9.Alpert B, Seligson D. Removal of asymptomatic bone plates used for orthognathic surgery and facial fractures. J Oral Maxillofac Surg 1996; 54:618–621. [DOI] [PubMed] [Google Scholar]
- 10.Bostman OM. Current concepts review absorbable implants for the fixation of fractures. J Bone Joint Surg 1991; 73:148–153. [PubMed] [Google Scholar]
- 11.Shikinami Y, Matsusue Y, Nakamura T. The complete process of bioresorption and bone replacement using devices made of forged composites of raw hydroxyapatite particles/poly L-lactide (F-u-HA/PLLA). Biomaterials 2005; 26:5542–5551. [DOI] [PubMed] [Google Scholar]
- 12.Schindler A, Harper D. Polylactide. II. Viscosity-molecular weight relationships and unperturbed chain dimensions. J Polym Sci 1979; 17:2593–2599. [Google Scholar]
- 13.Fischer EW, Sterzel HJ, Wegner G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z Z Polymere 1973; 251:980–990. [Google Scholar]
- 14.Bloebaum RD, Holmes JL, Skedros JG. Mineral content changes in bone associated with damage induced by the electron beam. Scanning 2005; 27:240–248. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi M, Muramatsu H, Sato M, et al. Surgical treatment of facial fracture by using unsintered hydroxyapatite particles/poly l-lactide composite device (OSTEOTRANS MX (®)): a clinical study on 17 cases. J Craniomaxillofac Surg 2013; 41:783–788. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, David O, Li Z-B. The use of resorbable plates in association with dental arch stabilization in the treatment of mandibular fractures in children. J Craniomaxillofac Surg 2013; 42:548–551. [DOI] [PubMed] [Google Scholar]
- 17.Matsusue Y, Niibayashi H, Aoki Y. Bone fusion using HA/PLLA composite, a material characterized by its excellent resorbability in the living body. J Orthop Surg 1999; 50:1405–1411. [Google Scholar]
- 18.Landes C, Ballon A, Tran A, et al. Segmental stability in orthognathic surgery: hydroxyapatite/Poly-l-lactide osteoconductive composite versus titanium miniplate osteosyntheses. J Craniomaxillofac Surg 2014; 42:930–942. [DOI] [PubMed] [Google Scholar]
- 19.Landes C, Ballon A, Ghanaati S, et al. Treatment of malar and midfacial fractures with osteoconductive forged unsintered hydroxyapatite and poly-L-lactide composite internal fixation devices. J Oral Maxillofac Surg 2014; 72:1328–1338. [DOI] [PubMed] [Google Scholar]
- 20.Matsusue Y, Yamamuro T, Yoshii S, et al. Biodegradable screw fixation of rabbit tibia proximal osteotomies. J Appl Biomater 1991; 2:1–12. [DOI] [PubMed] [Google Scholar]
- 21.Matsusue Y, Nakamura T, Iida H, Shimizu K. A long term clinical study on drawn poly-L-lactide implants in orthopaedic surgery. J Long Term Eff Med Implants 1997; 7:119–137. [PubMed] [Google Scholar]
- 22.Bergsma JE, de Bruijn WC, Rozema FR, et al. Late degradation tissue response to poly (L-lactide) bone plates and screws. Biomaterials 1995; 16:25–31. [DOI] [PubMed] [Google Scholar]
- 23.Sukegawa S, Kannno T, Kawai H, et al. Long-term bioresorption of bone fixation devices made from composites of unsintered hydroxyapatite particles and poly-L-lactide. J Hard Tissue Biol 2015; 24:219–224. [Google Scholar]
- 24.Shikinami Y, Okuno M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly L-lactide (PLLA). Part I: basic characteristics. Biomaterials 1999; 20:859–877. [DOI] [PubMed] [Google Scholar]
- 25.Stanton DC, Liu F, Yu JW, Mistretta MC. Use of bioresorbable plating systems in paediatric mandible fractures. J Craniomaxillofac Surg 2014; 42:1305–1309. [DOI] [PubMed] [Google Scholar]
- 26.Bayram B, Araz K, Uckan S, Balcik C. Comparison of fixation stability of resorbable versus titanium plate and screws in mandibular angle fractures. J Oral Maxillofac Surg 2009; 67:1644–1648. [DOI] [PubMed] [Google Scholar]
- 27.Esen A, AtaoÐlu H, Gemi L. Comparison of stability of titanium and absorbable plate and screw fixation for mandibular angle fractures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106:806–811. [DOI] [PubMed] [Google Scholar]
- 28.Bell BR, Kindsfater CS. The use of biodegradable plates and screws to stabilize facial fractures. J Oral Maxillofac Surg 2006; 64:31–39. [DOI] [PubMed] [Google Scholar]
- 29.Shikinami Y, Hata K, Okuno M. Ultra-high-strength resorbable implants made from bioactive ceramic particles/polylactide composites. Bioceramics 1996; 9:391–394. [Google Scholar]
- 30.Yasunaga T, Matsusue Y, Furukawa T, et al. Bonding behavior of ultrahigh strength unsintered hydroxyapatite particles/poly (L-lactide) composites to surface of tibial cortex in rabbits. J Biomed Mater Res 1999; 47:412–419. [DOI] [PubMed] [Google Scholar]
- 31.Suuronen R, Pohjonen T, Hietanen J, Lindqvist C. A 5-year in vitro and in vivo study of the biodegradation of polylactide plates. J Oral Maxillofac Surg 1998; 56:604–614. [DOI] [PubMed] [Google Scholar]
- 32.Törmälä P, Vasenius J, Vainionpää S, et al. Ultra-high-strength absorbable self-reinforced polyglycolide (SR-PGA) composite rods for internal fixation of bone fractures: in vitro and in vivo study. J Biomed Mater Res 1991; 25:1–22. [DOI] [PubMed] [Google Scholar]
- 33.Bostman OM, Hirvensalo E, Makinen J, Rokkanen P. Foreign body reactions to fracture fixation implants of biodegradable synthetic polymers. J Bone Jt Surg [Br] 1990; 72:592–596. [DOI] [PubMed] [Google Scholar]
- 34.Murthy AS, Lehman JA., Jr Symptomatic plate removal in maxillofacial trauma. A review of 76 cases. Ann Plast Surg 2005; 55:603–607. [DOI] [PubMed] [Google Scholar]
- 35.Rallis G, Mourouzis C, Papakosta V, et al. Reasons for miniplates removal following maxillofacial trauma: a 4-year study. J Craniomaxillofac Surg 2006; 34:435–439. [DOI] [PubMed] [Google Scholar]
- 36.Shikinami Y, Okuno M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly L-lactide (PLLA). Part II: practical properties of miniscrews and miniplates. Biomaterials 2001; 22:3197–3211. [DOI] [PubMed] [Google Scholar]
- 37.Bergsma EJ, Rozema FR, Bos RR, de Bruijn WC. Foreign body reactions to resorbable poly (L-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J Oral Maxillofac Surg 1993; 51:666–670. [DOI] [PubMed] [Google Scholar]
- 38.Kuvat SV, Cizmeci O, Bicer A, et al. Improving bony stability in maxillofacial surgery: use of osteogenetic materials in patients with profound (> or Z5 mm) maxillary advancement, a clinical study. J Plast Reconstr Aesthet Surg 2009; 62:639–645. [DOI] [PubMed] [Google Scholar]
- 39.Kim MG, Kim BK, Park JL, et al. The use of bioabsorbable plate fixation for nasal fractures under local anaesthesia through open lacerations. J Plast Reconstr Aesthet Surg 2008; 61:696–699. [DOI] [PubMed] [Google Scholar]
- 40.Kubota Y, Kuroki T, Akita S, et al. Association between plate location and plate removal following facial fracture repair. J Plast Reconstr Aesthet Surg 2012; 65:372–378. [DOI] [PubMed] [Google Scholar]
- 41.Givissis PK, Stavridis SI, Papagelopoulos PJ, et al. Delayed foreign-body reaction to absorbable implants in metacarpal fracture treatment. Clin Orthop Relat Res 2010; 468:3377–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shikinami Y, Matsusue Y, Nakamura T. The complete process of bioresorption and bone replacement using devices made of forged composites of raw hydroxyapatite particles/poly L-lactide (F-u-HA/PLLA). Biomaterials 2005; 26:5542–5551. [DOI] [PubMed] [Google Scholar]
- 43.Li S, Garreau H, Vert M. Structure-property relationships in the case of the degradation of massive poly (a-hydroxy acids) in aqueous media. Part 3. Influence of the morphology of poly (L-lactic acid). J Mater Sci Mater Med 1990; 1:198–206. [Google Scholar]


