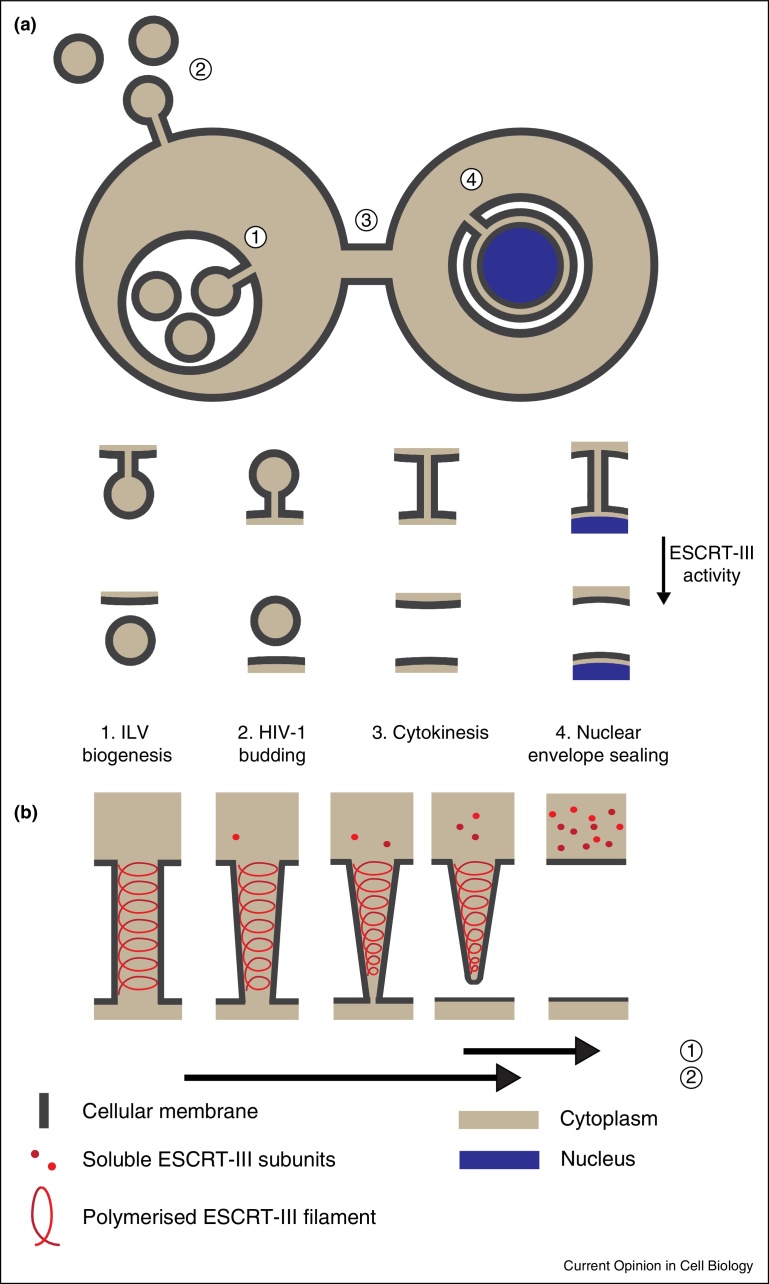
Figure 1.
Sites and models of ESCRT-III activity. (a) Cartoon depicting sites of topologically-equivalent membrane remodeling performed by the ESCRT-III complex. In all cases, ESCRT-III provides an activity allowing resolution of the membranous stalk with the concomitant separation of the two membranes previously connected by the stalk. This separation achieves release of ILVs (1), enveloped viruses such as HIV-1 (2), daughter cells (3) and separation of previously connected inner and outer nuclear membranes (4). Bottom schematic depicts membrane separation in each case achieved through ESCRT-III activity. (b) Models for ESCRT-III driven membrane fission. Filaments of polymerised ESCRT-III subunits are thought to assemble inside a membranous stalk, connecting two parental membranes. ESCRT-III assembly, either via the shape of the formed holo-polymer (dome model), or through constriction of the ESCRT-III filament (purse string model), narrows the stalk. This narrowing presumably makes it energetically more favourable to separate the membranes, rather than persist with membranes connected by a highly-curved, thin, membranous stalk. Models propose that the AAA-ATPase VPS4 acts either to tighten the filament, through sequential extraction of polymerised CHMPs (purse string model, VPS4 activity needed throughout remodeling event (1)) or through the induction of conformational changes in the CHMPs by direct interaction. Alternatively, membrane fission is accomplished through formation of the ESCRT-III holo-polymer (dome model, with VPS4 acting after fission to disassemble ESCRT-III filaments for subsequent rounds of assembly (2)). Period of VPS4 activity indicated by arrow.