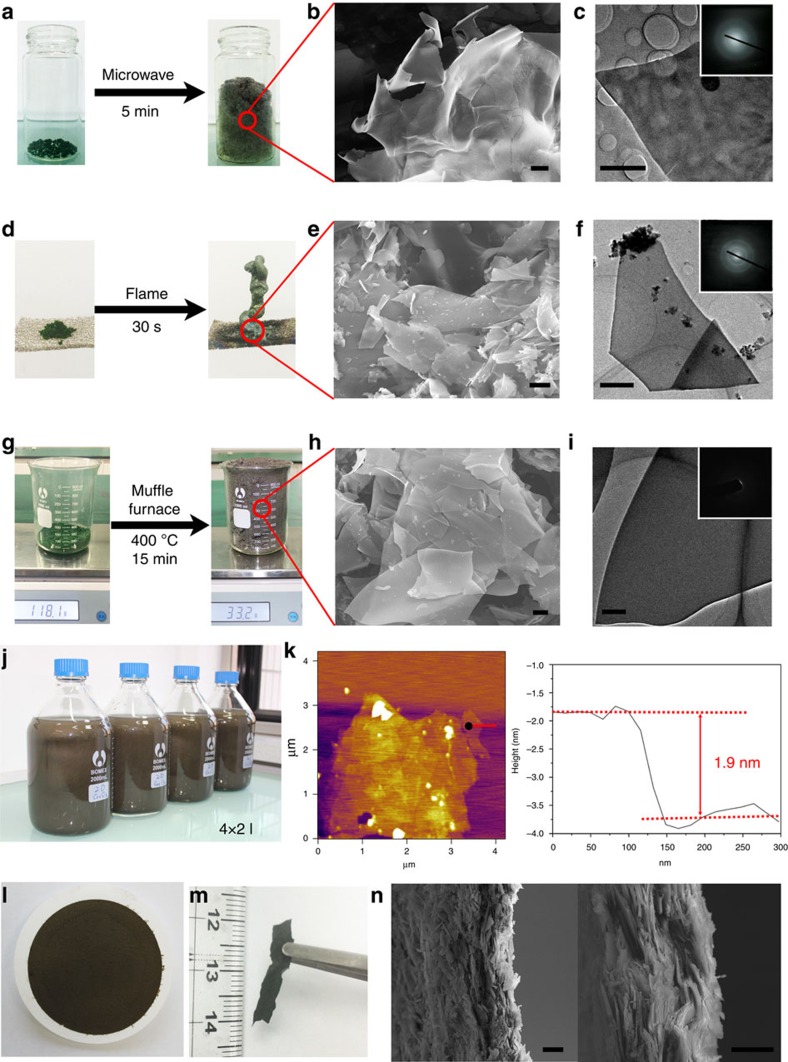
Figure 2. Nanosheets produced from chromium trichloride hexahydrate.
(a) A CrCl3·6H2O sample before (left of panel) and after (right of panel) microwave heating. (b) SEM and (c) TEM images (inset, corresponding electron diffraction pattern) correspond to the sample in a. (d) A CrCl3·6H2O sample before (left of panel) and after (right of panel) flame heating. (e) SEM and (f) TEM images (inset, corresponding electron diffraction pattern) correspond to the sample in d. (g) A CrCl3·6H2O sample before (left of panel) and after (right of panel) being heated in the muffle furnace. (h) SEM and (i) TEM images (inset, corresponding electron diffraction pattern) correspond to the CrCl3·6H2O sample in g. (j) A large quantity (8 l) of the 2D Cr2O3 dispersion solution. (k) AFM characterization of the Cr2O3 obtained by alcohol lamp heating. (l) The 2D Cr2O3 film produced by vacuum filtration. (m) The free-standing 2D Cr2O3 film. (n) Cross-sectional SEM images of the free-standing 2D Cr2O3 film. Scale bars, 2 μm (b,c), 5 μm (e), 500 nm (f,i) and 10 μm (h,n).