Summary
Objective
Our review analyses the studies that have specifically compared the association iDPP4/metformin with glimepiride/metformin, both in second line pharmacotherapy of type 2 diabetes mellitus (DM2).
Methods
Systematic literature review with a meta‐analysis of clinical trials comparing glimepiride with any iDPP4, both used together with metformin as a second line treatment of DM2. The effectiveness variables used were as follows: %HbA1c variation, fasting plasma glucose variation, patients achieving the therapeutic objective of HbA1c <7%, treatment dropouts due to lack of effectiveness and rescue treatments needed. The safety variables included were as follows: weight variation at the end of treatment; presentation of any type of adverse event; presentation of serious adverse events; patients who experienced any type of hypoglycaemia; patients who experienced severe hypoglycaemia; treatments suspended due to adverse effects; and deaths for any reason.
Results
Four studies met the inclusion criteria. The group treated with glimepiride showed better results in all effectiveness variables. Regarding safety variables, the main differences observed were in the greater number of cases with hypoglycaemia in the group treated with glimepiride, and the serious adverse events or treatment discontinuations due to these which occurred in slightly over 2% more cases in this group compared to the iDPP4 group. The remaining adverse events, including mortality, did not show any differences between both groups. The variation in the weight difference between groups (2.1 kg) is not considered clinically relevant.
Conclusions
A greater effectiveness is seen in the glimepiride/metformin association, which should not be diminished by slight differences in adverse effects, with absence of severe hypoglycaemia in over 98% of patients under treatment. The association of glimepiride/metformin, both due to cost as well as effectiveness and safety, may be the preferential treatment for most DM2 patients, and it offers a potential advantage in refractory hyperglycemic populations, tolerant to treatment.
Review criteria
Systematic literature review of clinical trials comparing glimepiride/metformin vs. any iDPP4/metformin.
Meta‐analysis has been realized using a fixed‐effects model according to basic criteria of Cochrane Handbook for Systematic Reviews of Interventions (V. 5.1.0). Sensibility analysis was carried out to explain statistical heterogeneity.
There have been analyzed basic outcomes established by the European Medicines Agency according to efficacy and safety (5 outcomes of each category).
Message for the clinic
All variables associated with effectiveness are consistently favourable to the combination of glimepiride with metformin.
Treatment discontinuations due to serious adverse events only occurred in slightly over 2% more cases in the glimepiride group. No severe hypoglycaemic episode was observed in 98% patients.
This treatment might be a preferential option for most DM2 patients who have not managed to achieve an adequate control with monotherapy
Introduction
The prevalence of type 2 diabetes mellitus (DM2) has been increasing in the last few decades, reaching pandemic proportions 1, 2 that is already overwhelming industrialised countries and is spreading to low and medium income countries, where an 80% mortality can be attributed to this disease 3. At the same time, the progressive nature of diabetes and its associated complications carry an important economic impact, both because of the use of healthcare resources as well as loss of productivity, which are frequently undervalued 2, 4, 5, 6.
Many, national and international, guidelines have been elaborated to standardise the complex management of this disease. Generally, and in addition to the permanent diet and physical exercise recommendations, the treatment guidelines suggest starting pharmacotherapy with metformin and, if the glycaemic objective (generally established at a glycosylated haemoglobin, HbA1c, concentration below 7% or even 6.5%) does not remain under control, then a second agent with a different action mechanism is added, among which a second generation sulfonylurea is usually recommended 7, 8, 9, 10, 11, 12.
Among antihyperglycaemic agents used in second line pharmacotherapy, we find the inhibitors of dipeptylpeptidase 4 (iDPP4) and second generation sulfonylureas.
Sulfonylureas have been known for decades, but because of the differences seen among the different ones in the group, they cannot be considered homogeneous 13, 14, 15, 16, 17. However, in the case of glimepiride, perhaps because of their most recent appearance, its distinguishing characteristics are frequently masked by the class effect of the group as a whole.
In clinical practice, the doctor does not prescribe a pharmaceutical group but a specific drug. As a consequence, the specific information must be available to allow him to distinguish one in particular among the different agents of each pharmacological family to personalise the treatment, as required by a patient‐centred approach 18. It is in this context which we consider that the assessment of the most used sulfonylureas requires individual studies to avoid confusion that could lead to an indiscriminate analysis of the drugs in the group. As a result, we have approached the study of glimepiride taking into account the growing interest this agent raises in the second line combination therapy 19 as well as the differences it presents compared to other drugs in the same group 20, 21, its favourable balance between effectiveness and safety 17, 22, together with its lower cost 23, 24.
The objective of this study was, through a systematic literature review, to compare the effectiveness and safety of glimepiride with any iDPP4 agent when both are used together with metformin in second line treatment of DM2.
Material and methods
The selection of studies was carried out applying the following inclusion criteria: randomised or quasi‐randomised clinical trials with a follow‐up of at least 12 weeks that include pre‐established variables on measures of effectiveness and safety, disaggregated by treatment group. Patients should be over 18 years of age and have a diagnosis of DM2, be on treatment with a stable dose of metformin for at least three months prior to the selection visit, and present an inadequate glycaemic control with HbA1C > 6.5%. Therefore, they should be considered for the second line pharmacotherapy and a second oral agent should be added, which could be either an inhibitor of dipeptylpeptidase 4 (iDPP4) or glimepiride. Included studies should compare an iDPP4 (alogliptin, linagliptin, saxagliptin, sitagliptin or vildagliptin) with glimepiride, both associated with metformin, as a second line pharmacotherapy. Non‐randomised clinical trials were excluded, as well as those randomised trials which included patients with a diagnosis of type 1 diabetes mellitus without presenting separate results for DM2; clinically relevant cardiovascular disease; myocardial infarction; ischemic attack in the previous 6 months or with abnormal laboratory results.
A literature search was carried out in Medline (through PubMed) until 31 December 2013. In addition, a manual search of the references from the articles obtained was carried out, as well as a search in the Cochrane Library database.
The following MeSH terms were used in a Boolean query which combined each of the separately searched iDPP4 (OR): alogliptin, sitagliptin, saxagliptin, vildagliptin and linagliptin, with metformin, and then combining them (AND) with the combination of glimepiride and metformin. No restriction of language or publication date was applied.
The selection of abstracts was independently carried out by two different researchers, and disagreements were resolved through discussion with a third researcher. Next, a thorough reading of the complete text of the articles selected was carried out to decide on their eligibility. The references obtained were imported to a Reference Manager version 12 file and two authors independently reviewed the studies to select those which met the inclusion criteria. Differences between the two reviewers were resolved by consensus with a third reviewer. Methodological quality and bias risk was assessed according to the Cochrane Collaboration criteria 25.
The result variables analysed were selected according to the basic criteria developed by the European Medicines Agency (EMA) 26. Thus, to evaluate effectiveness, we chose the change in percentage of glycosylated haemoglobin as the main variable (% HbA1c), and the following were considered as secondary variables: patients who achieved the therapeutic objective of HbA1c < 7%; change in fasting plasma glucose level (FPG); patients achieving the therapeutic objective of HbA1c < 7%, treatment dropouts because of lack of effectiveness; and rescue treatments needed. Safety variables included: weight variation at the end of treatment; presentation of any type of adverse event; presentation of serious adverse events; patients who experienced any type of hypoglycaemia; patients who experienced severe hypoglycaemia, that is, those cases that required assistance by a third party, whether it be professional or non‐professional assistance; treatments suspended because of adverse effects, and deaths for any reason.
The result variables were processed comparing their values at the end of the follow‐up period to the basal levels. Meta‐analysis was carried out using the statistics package stata version 12 (StataCorp LP, College Station, TX, 1984–2007) comparing the intervention group, those using iDPP4, with the active comparator, glimepiride, using a fixed‐effects model 27. Continuous variables were analysed using the difference in ponderated means (WMD), with the Mantel–Haenszel method and its corresponding 95% confidence interval. In the case of dichotomous variables, Odds ratio (OR) was calculated with its 95% confidence interval using the Peto method. The degree of inconsistency between study results was assessed using the I 2 statistic, using a value of I 2 > 50% as a limit for clinical relevance. Sensibility analysis was carried out to explain statistical heterogeneity. Given that the number of studies included in our review did not reach the minimum, 10, recommended in the Cochrane Manual 25 to carry out a funnel‐plot, this possibility was not considered.
Results
The study selection process is shown in Figure 1. The initial bibliographic search generated 53 potentially relevant references. After reading the abstracts of these references, 14 articles were selected for a complete text reading, and a further five were discarded for not meeting inclusion criteria 28, 29, 30, 31, 32, making a total of nine articles selected for inclusion in the quantitative systematic review 33, 34, 35, 36, 37, 38, 39, 40, 41. Out of these, five articles corresponded to primary studies 34, 37, 38, 40, 41; another is an intermediate publication of the same study by Ferrannini et al. 36 and another three are post‐hoc analyses of primary studies 33, 35, 39 which are excluded as they offer other composite end‐points or outcome variables which are different to those established in our inclusion criteria.
Figure 1.
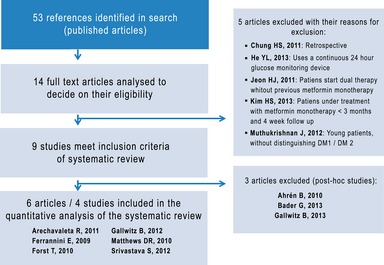
Bibliographic search diagram
The six selected articles correspond to five randomised, double blind, multi‐centre clinical trials, with the exception of Srivastava et al. 41 which is a single‐centre study and Forst et al. 37 which, although it is randomised in both arms, only the iDPP4 arm was blinded. The follow‐up periods varied between 12 and 104 weeks and four studies 34, 36, 38, 40 were funded by the pharmaceutical industry.
Quality of studies
All articles suffer from uncertainties which could be the cause of bias, thus: four articles do not mention how sample size was calculated to assure statistical power 34, 36, 38, 41; two 37, 38 do not mention if patients were receiving concomitant treatments or their description and another article 40 presents a dropout rate > 30% when 20% was estimated when sample size was calculated. Taking this into account, the methodological quality of the remaining six articles was assessed, as represented in Figure 2, after which we decided to exclude the high risk of bias articles 40, 41 and replace Matthews 2010 40 by the one by Ferrannini et al. 36 as it belonged to the same study but presented better quality indicators and provided results with a 52 week follow‐up.
Figure 2.
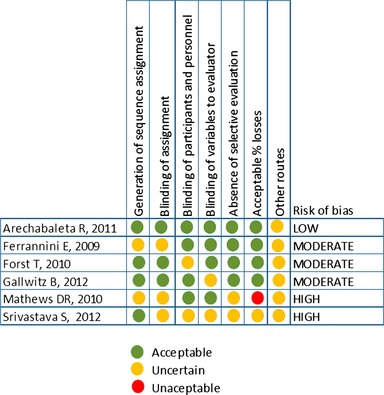
Assessment of the methodological quality of studies
All together, the selected articles included results from 5637 patients, mainly Caucasian (81%) and male (54%), with a mean age of 58 ± 9.4 years, a mean basal weight of 86.8 ± 17.1 kg, and an average BMI of 31.2 ± 4.9. The mean progression period of diabetes was 5.8 ± 4.5 years; mean basal HbA1c was 7.5% ± 0.7%, and the average FPG was 9.0 ± 2.2 mmol/l. The iDPP4 used in the studies was sitagliptin 34, vildagliptin 36 and linagliptin 37, 38, the results of which are processed together to explore a class effect. The basal characteristics of the patients are described in Table 1 where, among the differences found between them, we would like to point out the following: three of the articles have a non/inferiority design 34, 36, 38, 40 and two of them mention the possibility of introducing rescue medication 36, 38, 40.
Table 1.
Basal characteristics of articles and included patients
| Author/Year/Country/Study | Treatment groups | N | Study duration (weeks) | Age (years), mean ± SD) | Sex (% male) | T2DM duration (years), mean ± SD | Basal HbA1c (%), mean ± SD | FPG (mmol/l), mean ± SD | Basal weight (kg), mean ± SD | Basal BMI (kg/m2), mean ± SD | a) Rescue medication (%) b) Concomitant medication c) Adherence |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Arechabaleta, 2011 USA Non‐inferiority design NCT00701090 |
Sitagliptin (100 mg/day) Glimepiride (1–6 mg/day) Mean dose: 2.1 mg/day Metformine (≥ 1500 mg/day) |
516 519 |
30 |
56.3 ± 9.7 56.2 ± 10.1 |
55 54 |
6.8 ± 4.6 6.7 ± 4.8 |
7.5 ± 0.7 (6.0–10.2) 7.5 ± 0.8 (6.1–10.3) |
8.0 ± 1.8 8.1 ± 1.9 |
80.6 ± 15.2 82.0 ± 16.7 |
29.7 ± 4.5 30.2 ± 4.4 |
a) No b) Yes c) Yes (97%) |
|
Ferrannini, 2009 France Non‐inferiority design NCT00106340 |
Vildagliptin (50 mg bid) Glimepiride (2–6 mg/day) Mean dose: 4.5 mg/day Metformine Mean dose: 1898 mg/day |
1396 1393 |
52 |
57.5 ± 9.06 57.46 ± 9.28 |
53 54 |
5.7 ± 5.2 5.8 ± 5.0 |
7.3 ± 0.6 7.3 ± 0.7 |
9.2 ± 2.3 9.2 ± 2.2 |
89.01 88.62 |
31.8 ± 5.3 31.7 ± 5.3 |
a) 5.1 vs. 3.7 Pioglitazone if HbA1c>8% b) Yes (93%) c) ND |
|
Forst 2010 Germany NCT00309608 |
Linagliptin (1 mg/day) Linagliptin (5 mg/day) Linagliptin (10 mg/day) Glimepiride (1–3 mg/day) Metformine (≥ 1500 mg/day) |
65 66 66 65 |
12 |
59.2 ± 8.4 59.6 ± 9.8 61.8 ± 8.8 59.4 ± 9.9 |
55 63 |
7.5 ± 6.8 6.7 ± 5.9 |
8.2 ± 0.7 8.5 ± 0.8 8.4 ± 0.7 8.2 ± 0.7 |
10.1 ± 2.3 10.5 ± 2.4 10.5 ± 2.4 10.0 ± 2.2 |
92.5 ± 16.9 90.7 ± 14.2 89.9 ± 16.3 90.5 ± 15 |
32.3 ± 4.3 31.7 ± 4.5 31.7 ± 4.5 31.5 ± 4.2 |
a) No b) ND c) ND |
|
Gallwitz 2012 Germany Non‐inferiority design NCT00622284 |
Linagliptin (5 mg qd) Glimepiride (1–4 mg qd) Metformine (≥ 1500 mg/day) |
776 775 |
104 |
59.8 ± 9.4 59.8 ± 9.4 |
60 61 |
≤ 1 year: 50 (7%)* L 58 (8%)* G 1 ≤ years ≤ 5: 316 (41%)* L 291 (39%)* G ≥ 5 years: 398 (52%)* L 406 (54%)* G |
7.7 ± 0.9 7.7 ± 0.9 |
9.1 ± 2.4 9.2 ± 2.3 |
86.0 ± 17.6 87.0 ± 16.7 |
30.2 ± 4.8 30.3 ± 4.6 |
a) 25 vs. 21 p = 0.117; Pioglitazone if HbA1c > 8.5% b) Yes (< 91%) c) Yes (98%) |
bid, twice at day; BMI, body mass index; FPG, fasting plasma glucose; N, number of patients; ND, Not described in the study; SD: standard deviation. *Number and (%) of patients under such condition (L = Linagliptin; G = Glimepiride).
The criteria defining a failure in glycaemic control of the previous treatment also varied between studies. In two studies 34, 36, the treatment prior to inclusion consisted exclusively of metformin monotherapy, while in another two studies 37, 38, patients were recruited after having received metformin treatment either as monotherapy or associated with another oral antidiabetic medication.
Finally, there are also differences in the maximum doses of glimepiride established which vary between 3 37 and 6 mg/day 34, 36
Effectiveness
Reduction in HbA1c levels
The combined analysis of HbA1c variation after treatment in the four articles selected 34, 36, 37, 38 includes the results of the observations after the different follow‐up periods and shows that patients treated with glimepiride have a 12% greater reduction compared with those treated with iDPP4, WMD –0.12 (CI: −0.16, −0.07), as can be seen in Figure 3.
Figure 3.
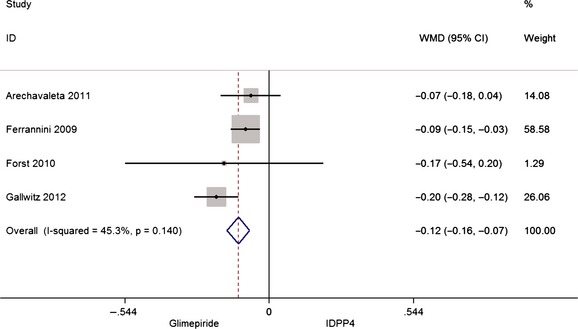
Meta‐analysis of HbA1c (%) reduction after treatment
Proportion of patients achieving the objective of HbA1c < 7%
Three of the studies selected 34, 36, 38 use the proportion of patients who achieve the objective of HbA1c < 7% as a secondary effectiveness outcome, and two of them 34, 38 also add those patients with HbA1c < 6.5%. The meta‐analysis of the results from the first studies, shows a favourable result for glimepiride, OR: 1.14 (CI: 1.01, 1.28; I 2 = 13.5%).
FPG variation
The variation in FPG is studied in three studies 34, 36, 38 and its combined analysis shows that the association of glimepiride/metformin produces a reduction 0.21 mmol/l greater than with iDPP4/metformin (I 2 = 17.4%).
Dropouts because of lack of effectiveness
Four studies 34, 36, 37, 38 analysed the number of dropouts in each group because of the lack of effectiveness. Results show that there are significantly fewer dropouts, 50%, in the glimepiride group compared with the iDPP4 group, as can be seen in Figure 4.
Figure 4.
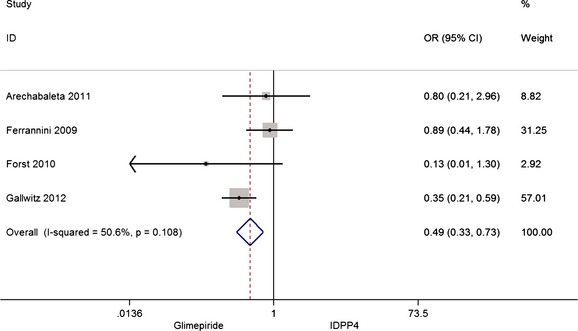
Risk of dropout because of the lack of effectiveness
Need for rescue treatments
Two studies 36, 38 analyse the number of rescue treatments needed with another drug, because of lack of effectiveness of the treatments under study. The combined analysis of this variable shows that in the group treated with glimepiride/metformin, the risk of needing rescue treatments is 20% less than in the iDPP4/metformin group (OR: 0.80, 95% CI: 0.65, 0.99; I 2 = 0.0%).
Safety
Weight variation
Table 2 summarizes basal body mass index 42 and weight in the different treatment groups, together with the variations in weight experienced in each group, expressed as an absolute value (kg) and as a proportion (%). The greatest weight reduction, that corresponds to a difference of 1.63% from the basal level, is seen with the treatment of linagliptine after 104 weeks, while the greatest increase, which is 1.76% compared with the basal weight, is observed after 52 weeks of treatment with glimepiride.
Table 2.
Weight variation (▵) in the different treatment groups with basal BMI and weight
| Author | Glimepiride | iDPP4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment (weeks) | BMI (kg/m2) | Weight (kg) | ΔWeight (kg) | ΔWeight (%) | BMI (kg/m2) | Weight (kg) | ΔWeight (kg) | ΔWeight (%) | |
| Forst (2010) | 12 | 31.5 | 91 ± 15 | 0.73 | 0.81 | 31.7 | 91 ± 14 | −0.57 | −0.63 |
| Arechavaleta (2011) | 30 | 30.2 | 82 ± 17 | 1.2 | 1.46 | 29.7 | 81 ± 15 | −0.8 | −1 |
| Ferrannini (2009) | 52 | 31.7 | 89 | 1.56 | 1.76 | 31.8 | 89 | −0.23 | −0.26 |
| Gallwitz (2012) | 104 | 30.3 | 86 ± 18 | 1.3 | 1.49 | 30.2 | 87 ± 17 | −1.4 | −1.63 |
Given that weight variation can occur in both directions: increase and decrease, in the combined analysis of this variable, the differences produced between both treatment groups have been processed. The overall difference between the increase in weight experienced in the groups treated with glimepiride and the decrease in weight observed in those treated with iDPP4 is 2.1 kg (95% CI: 1.78, 2.24; I 2 = 74.3%).
Adverse effects
Four studies 34, 36, 37, 38 analysed the number of adverse effects in each group. The combined analysis of the number of patients experiencing adverse effects of any severity shows a high proportion. Over 70%, is seen in both groups: 71.9% in patients treated with iDPP4 and 78.3% in those treated with glimepiride, which means that out of one hundred patients who receive each of these treatments, in the glimepiride group there are six cases more experiencing adverse events than in the iDPP4 group (95% CI: 1.29, 1.67; I 2 = 14.0%).
As indicated, these figures include all type of adverse effects, including the severe ones which are examined later on. However, the articles analysed mention several adverse effects which are produced with a frequency ≥ 5%, and include the following: headaches, cough, nasofaringitis, urinary infection, musculoskeletal and gastrointestinal disorders, flu and hypoglycaemia. Except this last one, the others do not show differences between both treatment groups.
The same articles 34, 36, 37, 38 include also information about the number of patients who present serious adverse effects including episodes of severe hypoglycaemia. The combined analysis shows greater proportion in the group treated with glimepiride, as can be seen in Figure 5. However, analysis of the crude figures reflects a much smaller difference: 9.1% in the group treated with iDPP4 and 11.2% in the group treated with glimepiride.
Figure 5.
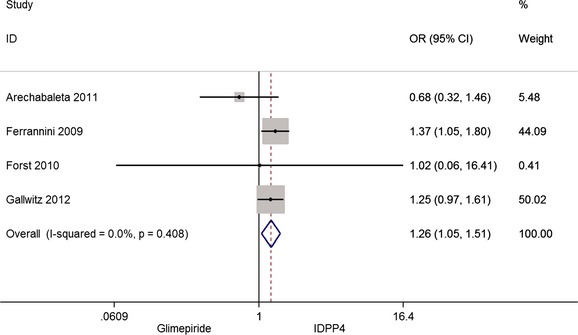
Risk of serious adverse effects
Hypoglycaemia
Four articles 34, 36, 37, 38 analyse this variable and results show that in patients treated with glimepiride there are more cases of patients suffering from any type of hypoglycaemia than in those treated with IDPP4: OR: 5.07 (95% CI: 4.33, 5.93; I 2 = 59.2%), although this difference is mainly due to the cases of mild or moderate hypoglycaemia, as can be seen by the next analysis of severe hypoglycaemia.
Three of the articles 34, 36, 38 have separately analysed the hypoglycaemic episodes according to their severity. According to the combined analysis of this variable, the magnitude of the effect is greater in the glimepiride group: OR: 5.57 (95% CI: 2.79, 10.34; I 2= 0.0%) and corresponds to 0.1% of patients treated with iDPP4 who suffer some episode compared to 1.2% in the group treated with glimepiride.
Discontinuation caused by adverse events
Discontinuation caused by adverse events is analysed in four studies 34, 36, 37, 38 and their combined analysis shows greater proportion in the group treated with glimepiride, OR: 1.45 (95% CI: 1.17, 1.81; I 2 = 69.2%). This variable is important in reflecting the effective clinical relevance of adverse events in each of the treatments analysed as, without undermining the combined analysis, the proportion of discontinuations because of adverse events show only a difference of two more patients suffering from these out of every hundred patients treated with glimepiride: 7.3% compared to 5.2% in cases treated with iDPP4.
Deaths for any reason
The combined analysis does not show any difference in the number of deaths because of any cause between both groups (Figure 6), with 0.2% deaths in the treatment with iDPP4 and 0.3% with glimepiride.
Figure 6.
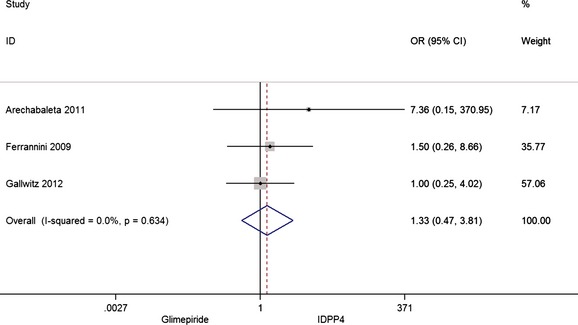
Risk of death for any season
Discussion
Composite end‐points
The clinical application of composite end‐points is discussed due to the heterogeneity among their components and the relative influence of each of them on treatment 43, 44, 45, 46, 47, which is the reason why in our review we have picked articles that express their results in simple primary variables, excluding those that were extension studies or those expressing their results as composite end‐points 33, 35, 39, that can offer an excessive simplification of evidence and result in mistakes in clinical practice, where the individual needs of each patient, defined by their specific characteristics such as age, glycaemic level, response and tolerance to treatment or associated morbidities, prevail 18, 48.
Glycaemic control
The main effectiveness variable presented in the four studies analysed is the variation in HbA1c with respect to basal levels. In those studies with greater number of patients, the average values of HbA1c in each treatment group, vary between 7.30 ± 0.65 in the group treated with glimepiride by Ferrannini et al. 36 and 7.7 ± 0.9 in both groups treated with linagliptine and glimepiride by Gallwitz et al. 38. However, this value increases to 8.5 ± 0.8 in the 66 patients treated with 5 mg/day of linagliptine by Forst et al. 37. The weight of this work in the overall analysis of this end‐point, is only 1.29%, and it studies the effects after only 12 weeks of treatment with three different doses of linagliptine, which may explain the amplitude of the range.
As can be seen in fig. 3, the study by Gallwitz, with a 2 year follow‐up and significant adherence, is the one to offer a greater magnitude of effect (20%) in the reduction in HbA1c concentration in favour of glimepiride treatment. Together with the proven effectiveness of the medication, we can also relate this result to the favourable adherence of patients treated that is a result of the careful therapeutic approach taken.
The percentage of patients achieving the therapeutic objective of HbA1c < 7% also shows a favourable global effect estimator in favour of glimepiride, despite the selection bias that can be attributed to the small basal concentration in the three studies analysed, as all included great proportions of patients with values of HbA1c between 6.5% and 7%, that are only quantified by Arechavaleta et al. 34 and Gallwitz et al. 38, as 22% and 23% in the groups treated with iDPP4 and 24% and 21% in the glimepiride treated groups.
Fasting plasma glucose levels offer an analogous reflection on possible biases as, except for the small study by Forst et al. 37, most patients treated in the different groups present FPG levels around 9 mmol/l and have had diabetes for approximately 6 years. In any case, the combined analysis, which is quite consistent, shows a significantly greater global estimator for glimepiride in 0.21 mmol/l, compared with iDPP4.
The cases of treatment discontinuation resulting from the lack of effectiveness, 1.2% vs. 2.4%, as well as the need to start rescue treatments, 11% vs. 13.1%, offer a favourable result for glimepiride treatment.
Effect on weight
The study of oral antidiabetics has paid significant attention to their effect on patient′s weight. In our study, we see that weight variations, in both directions, seen in the different groups are small, and clinically not relevant as, although there are no totally accepted thresholds to define a minimum weight change that can be considered significant, the published literature estimates decreases between 5% and 10% 7, 49, very far from those seen in our review, where the combined analysis of weight reductions observed with iDPP4 and the increases observed with glimepiride only show a difference of 2 kg between both treatment groups.
Ethnic differences are not always well reflected in the international weight classification based on Body Mass Index 42, as has been proposed in different populations such as the Asian 50. In the German population, an increase in mortality risk by any cause has been observed in obese people (BMI ≥ 36), both in the general population and in the diabetic one, but not in the overweight population 51. This trend in diabetic population has recently been confirmed, with the observation of a decrease in HbA1c concentration and decreased mortality risk in overweight states (25 ≤ BMI ≤ 35), defining what has been called ‘U figure’ 42, 52, 53.
The controversy arisen by the so‐called ‘obesity paradox’ has not yet reached an unanimous conclusion 54, 55 and still brings up methodological discussions which even suggest that the current obesity classification criteria are not enough 56, 57, 58, 59, 60. In addition, the differences between the effective weight variation and that perceived by patients were added to this controversy 61.
Hypoglycaemia
Hypoglycaemia has an important impact in the management of DM2 patients, and the complexity of this phenomenon itself requires careful assessment for several reasons such as: the scarce consistency in its definition; the continuous changes that have taken place in defining therapeutic objectives, as well as the duration itself of diabetes and the degree of insulin deficiency, in addition to the inter‐individual variability itself, as some patients experience repetitive hypoglycaemic episodes and others only occasionally 62. At the same time, hypoglycaemic episodes are related to the HbA1c objective which, together with the overall treatment, needs to be personalised as a function of the patient′s characteristics and risk factors such as: intensive control and duration of diabetes, hypoglycaemia history, cognitive state, comorbidities or poli‐medication 12, 63, 64. To this, we must add the fact that patients more vulnerable to a repetition of these episodes are more prone to loss of adherence 65 which, in any case, must be fought against with greater personalisation in patient training and care 66, 67 or ultimately, with treatment revision, as adequate glycaemia monitoring and subsequent adjustment of medication, diet, and physical activity, substantially contributes to the prevention of hypoglycaemic episodes 12.
In our analysis, the main safety variable used is the number of patients who have suffered a severe hypoglycaemia episode, defined as an episode which requires assistance by another person, be it professional or not 68, allowing us to manage more standardised data and with a greater healthcare impact.
However, when analysing overall hypoglycaemia, mild or moderate hypoglycaemia was also included, despite the ambiguity that can arise in declaring symptomatic episodes of varying intensity and frequency. Although more frequent in glimepiride treatment, it has proven to be of little clinical relevance, given the small number of treatment discontinuations because of adverse effects.
In the articles analysed, numerous patients have basal HbA1c levels which are under 7% that is the basic glycaemic objective of the studies themselves and the one proposed in general in the main guidelines, which also often suggest objectives such as HbA1c < 6.5% or HbA1c < 8%, depending on the specific characteristics of each patient 7, 10, 18, 69, 70, 71, 72, accepting the difficulty, in general, of achieving a HbA1c < 7% 18.
In any case, we must emphasise that the number of patients who experience severe hypoglycaemic episodes only reaches 1.2% of all patients treated with glimepiride.
On the other hand, the fact that longer treatments present severe hypoglycaemic episodes in 0.72% 36 and 1.55% 38 of subjects treated with glimepiride through 52 and 104 weeks, respectively, compared with the 2.12% seen in the study by Arechavaleta et al. 34, of only 30 weeks, suggests a possible effect of patient adaptation to treatment
The combined analysis of patients who experience adverse effects, of any type or any severity, is greater in the group treated with glimepiride, resulting from the episodes of hypoglycaemia. However, when these are excluded, the mild or moderate adverse effects with a frequency of ≥ 5, do not show differences between groups.
As the combined analysis has revealed, the number of patients who experience serious adverse effects, including serious hypoglycaemia, is greater in the group treated with glimepiride. However, crude data show this greater incidence is limited, in practice, to 2 of every 100 patients treated: 9.1% in the iDPP4 group and 11.2% in the glimepiride group. This similarity is maintained when the number of treatments discontinued because of adverse effects is assessed: 5.2% in the iDPP4 group and 7.3% in the glimepiride one.
Mortality
Significant differences between the groups have not been observed.
Study limitations
This study presents the limitations associated with, to a large degree, the lack of original articles and their objectives themselves, directed at showing a better tolerance of iDPP4, without a decrease in effectiveness. Thus, apart from the work by Forst et al. 37, which only has a 12 week follow‐up and with low weight in the meta‐analysis, the studies analysed have a non‐inferiority design, with margins of HbA1c established at 4% 34 in one of them and 3.5% in another 38, both above the 3% margin considered acceptable by the EMA 26, which is why we believe the initial assertion of ‘non‐inferiority’ must be interpreted with caution. In any case, the analysis has been done on outcome variables which are clearly quantified in the original studies, without carrying out any assignments or estimation approaches.
Conclusions
Our review on effectiveness and safety analyses the studies that have specifically compared the association glimepiride/metformin with iDPP4/metformin, both in second line pharmacotherapy of DM2 because of the distinguishing characteristics of this sulfonylurea, which, perhaps because of their most recent appearance, are frequently masked by the class effect of the group as a whole.
The association of iDPP4 or sulfonylureas with metformin has generally been compared on a non‐inferiority basis and greater tolerance of the first. We believe the results from non‐inferiority studies which apply margins above those considered acceptable by EMA must be interpreted with caution. In any case, the response to all effectiveness variables related to antihyperglycaemic treatment effect such as: reduction in HbA1c, proportion of patients who achieve HbA1c < 7%, and decrease in FPG, are consistently favourable to glimepiride. These results are complementary to those of treatment discontinuation because of the lack of effectiveness and start of rescue treatment with other drugs, which at the same time are also favourable to the association glimepiride/metformin.
The differences in weight variation, in both directions, experienced in the treatments with each of the agents studied is 2 kg, very far from magnitudes found in the literature to show clinical relevance, thus we do not consider these differences to be clinically important.
The analysis of adverse effects with a frequency of ≥ 5%, do not show differences between groups, except for mild or moderate hypoglycaemia, more common in those treated with glimepiride. In both treatment groups, general adverse effects are observed in more than 70% of patients treated: 71.9% with iDPP4 and 78.3% with glimepiride. This difference is reduced when analysing the serious adverse effects, which only occur in two patients more out of every 100 patients treated with glimepiride. Severe hypoglycaemia, despite being greater in the group treated with glimepiride, only occurs in 1.2% of patients with this treatment. The clinical relevance of the overall effects can be seen in the number of patients who had to discontinue treatment because of the adverse effects, which only shows a difference between treatments of two patients for every one hundred: 7.3% with glimepiride compared to 5.2% with iDPP4.
The fact that no severe hypoglycaemic episode was observed in over 98% patients offers a broad margin for use of glimepiride /metformin which, as occurs with any type of treatment, does not mean that treatment should not be checked and revised in those patients who show intolerance.
In summary, there is a greater effectiveness in the glimepiride/metformin association which should not be undermined by slight differences in adverse effects. The glimepiride/metformin association, both because of effectiveness and safety as well as cost, could be the preferential treatment for most DM2 patients who have not managed to achieve an adequate control with monotherapy, and it offers a potential advantage in refractory hyperglycaemic populations, tolerant to treatment.
Acnowledgements
This study has been carried out in the framework of a research agreement between Laboratories Silanes and the Institute of Health Carlos III (Spanish Governmental Institute of Biomedical Research). Authors would like to thank Mr. Vicente D__z del Campo (Spanish National Library of Health Sciences) his help in the provision of bibliographical material, and Ms. Ana Zurdo‐Manso her help in the electronic design of images.
Author contributions
J.M. Amate, was involved in the concept, design, data interpretation, the resolution of differences between reviewers and coordination of the study. T. López‐Cuadrado, was involved in the quality assessment and review of articles, data extraction and statistical analysis. N. Almendro‐Motos, was involved in the bibliographic search, quality assessment of articles, review of articles and data extraction. C. Bouza, was involved in the study design, the resolution of differences between reviewers and data interpretation. Z. Saz‐Parkinson, was involved in the critical review of the text and its translation into English. R. Rivas‐Ruiz and J. Gonzalez‐Canudas were involved in the study design. All authors participated in the drafting of this manuscript and its final approval.
Disclosure All authors are employed of the governmental institutions that have been indicated. Apart from Jorge Gonzalez‐Canudas MD, which is part‐time employed of Labs. Silanes, authors have not financial interest in any of the drugs discussed in this study.
References
- 1. Danaei G, Finucane MM, Lu Y et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2.7 million participants. Lancet 2011; 378(9785): 31–40. [DOI] [PubMed] [Google Scholar]
- 2. International Diabetes Federation . Diabetes Atlas, 6th edn ISBN: 2‐930229‐85‐3: International Diabetes Federation, 2013. [Google Scholar]
- 3. WHO . 10 facts about diabetes. http://www.whoint/features/factfiles/diabetes/facts/en/index7.html (accessed February 18, 2014).
- 4. American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013; 36(4): 1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breton MC, Guenette L, Amiche MA, Kayibanda JF, Gregoire JP, Moisan J. Burden of diabetes on the ability to work: a systematic review. Diabetes Care 2013; 36(3): 740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanavos P, van den Aardweg S, Schurer W. Diabetes expenditure, burden of disease and management in 5 EU countries. LSE Health, London School of Economics, 2012.
- 7. American Association of Clinical Endocrinologist . AACE Diabetes Care Plan Guidelines. Endocr Pract 2011; 17 (Suppl. 2): 1–53 [DOI] [PubMed] [Google Scholar]
- 8. Asociación Latinoamericana de Diabetes . Guías ALAD sobre el Diagnóstico y Tratamiento de la Diabeets Mellitus Tipo 2 con Medicina Basada en la Evidencia, 2013.
- 9. Bailey T. Options for combination therapy in type 2 diabetes: comparison of the ADA/EASD position statement and AACE/ACE algorithm. Am J Med 2013; 126(9 Suppl. 1): S10–20. [DOI] [PubMed] [Google Scholar]
- 10. Centre for Clinical Practice at NICE . Type 2 Diabetes: Newer Agents for Blood Glucose Control in Type 2 Diabetes. London, UK: National Institute for Health and Clinical Excellence, 2009. [PubMed] [Google Scholar]
- 11. International Diabetes Federation . IDF Diabetes Atlas Update 2012, 2012. [PubMed]
- 12. Riethof M, Flavin P, Lindvall B et al. Diagnosis and Management of Type 2 Diabetes Mellitus in Adults. http://bit.ly/Diabetes0412. Updated April 2012. Accessed on 9th April, 2014, Institute for Clinical Systems Improvement, 2012. [Google Scholar]
- 13. del Prato PS, Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism 2006; 55(5 Suppl. 1): S20–7. [DOI] [PubMed] [Google Scholar]
- 14. Harrower AD. Comparative tolerability of sulphonylureas in diabetes mellitus. Drug Saf 2000; 22(4): 313–20. [DOI] [PubMed] [Google Scholar]
- 15. Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005; 65(3): 385–411. [DOI] [PubMed] [Google Scholar]
- 16. Magnusson NE, Dyrskjot L, Grimm D, Wehland M, Pietsch J, Rungby J. Gene networks modified by sulphonylureas in beta cells: a pathway‐based analysis of insulin secretion and cell death. Basic Clin Pharmacol Toxicol 2012; 111(4): 254–61. [DOI] [PubMed] [Google Scholar]
- 17. Makkar B, Gupta D, Gainda A. Clinical Trials to Clinical Practice: Role of Sulfonylureas in Today's Practice In: Muruganathan A, ed. Medicine Update. Jaypee Brothers Medical Publishers PVT LTD: New Delhi, 2013: 393–8. [Google Scholar]
- 18. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012; 55(6): 1577–96. [DOI] [PubMed] [Google Scholar]
- 19. Pareek A, Chandurkar NB, Salkar HR, Borkar MS, Tiwari D. Evaluation of efficacy and tolerability of glimepiride and metformin combination: a multicentric study in patients with type‐2 diabetes mellitus, uncontrolled on monotherapy with sulfonylurea or metformin. Am J Ther 2013; 20(1): 41–7. [DOI] [PubMed] [Google Scholar]
- 20. Davis SN. The role of glimepiride in the effective management of type 2 diabetes. J Diabetes Complications 2004; 18(6): 367–76. [DOI] [PubMed] [Google Scholar]
- 21. Briscoe VJ, Griffith ML, Davis SN. The role of glimepiride in the treatment of type 2 diabetes mellitus. Expert Opin Drug Metab Toxicol 2010; 6(2): 225–35. [DOI] [PubMed] [Google Scholar]
- 22. Korytkowski MT. Sulfonylurea treatment of type 2 diabetes mellitus: focus on glimepiride. Pharmacotherapy 2004; 24(5): 606–20. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, McCoy RG, Mason JE, Smith SA, Shah ND, Denton BT. Second‐line agents for glycemic control for type 2 diabetes: are newer agents better? Diabetes Care 2014; 37(5): 1338–45. [DOI] [PubMed] [Google Scholar]
- 24. Klarenbach S, Cameron C, Singh S, Ur E. Cost‐effectiveness of second‐line antihyperglycemic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. CMAJ 2011; 183(16): E1213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane Collaboration, 2013.
- 26. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus, CPMP/EWP/1080/00 Rev. 1., Committee for Medicinal Products for Human Use (CHMP), 2012. [Google Scholar]
- 27. Sterne JA, Bradburn M, Egger M. Meta‐analysis in stata In: Egger M, Smith G, eds. Systematic Reviews in Health Care: Meta‐Analysis in Context, 2nd edn London: BMJ Publishing Group, 2008: 347–369. [Google Scholar]
- 28. Chung HS, Lee MK. Efficacy of sitagliptin when added to ongoing therapy in Korean subjects with type 2 diabetes mellitus. Diabetes Metab J 2011; 35(4): 411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeong KH, Yoo BK. The efficacy and safety of liraglutide. Int J Clin Pharm 2011; 33(5): 740–9. [DOI] [PubMed] [Google Scholar]
- 30. Muthukrishnan J, Dawra S, Marwaha V, Bishnoi JS, Narayanan CS. Diabetes mellitus in the young: gliptins or sulfonylurea after metformin? Indian J Endocrinol Metab 2012; 16(Suppl. 2): S474–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He YL, Foteinos G, Neelakantham S et al. Differential effects of vildagliptin and glimepiride on glucose fluctuations in patients with type 2 diabetes mellitus assessed using continuous glucose monitoring. Diabetes Obes Metab 2013; 15(12): 1111–9. [DOI] [PubMed] [Google Scholar]
- 32. Kim HS, Shin JA, Lee SH et al. A comparative study of the effects of a dipeptidyl peptidase‐IV inhibitor and sulfonylurea on glucose variability in patients with type 2 diabetes with inadequate glycemic control on metformin. Diabetes Technol Ther 2013; 15(10): 810–6. [DOI] [PubMed] [Google Scholar]
- 33. Ahren B, Foley JE, Ferrannini E et al. Changes in prandial glucagon levels after a 2‐year treatment with vildagliptin or glimepiride in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care 2010; 33(4): 730–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arechavaleta R, Seck T, Chen Y et al. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double‐blind, non‐inferiority trial. Diabetes Obes Metab 2011; 13(2): 160–8. [DOI] [PubMed] [Google Scholar]
- 35. Bader G, Geransar P, Schweizer A. Vildagliptin more effectively achieves a composite endpoint of HbA(1)c <7.0% without hypoglycaemia and weight gain compared with glimepiride after 2 years of treatment. Diabetes Res Clin Pract 2013; 100(3): e78–81. [DOI] [PubMed] [Google Scholar]
- 36. Ferrannini E, Fonseca V, Zinman B et al. Fifty‐two‐week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab 2009; 11(2): 157–66. [DOI] [PubMed] [Google Scholar]
- 37. Forst T, Uhlig‐Laske B, Ring A et al. Linagliptin (BI 1356), a potent and selective DPP‐4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes. Diabet Med 2010; 27(12): 1409–19. [DOI] [PubMed] [Google Scholar]
- 38. Gallwitz B, Rosenstock J, Rauch T et al. 2‐year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double‐blind, non‐inferiority trial. Lancet 2012; 380(9840): 475–83. [DOI] [PubMed] [Google Scholar]
- 39. Gallwitz B, Rosenstock J, Emser A, von EM, Woerle HJ. Linagliptin is more effective than glimepiride at achieving a composite outcome of target HbA(1)c <7% with no hypoglycaemia and no weight gain over 2 years. Int J Clin Pract 2013; 67(4): 317–21. [DOI] [PubMed] [Google Scholar]
- 40. Matthews DR, Dejager S, Ahren B et al. Vildagliptin add‐on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2‐year study. Diabetes Obes Metab 2010; 12(9): 780–9. [DOI] [PubMed] [Google Scholar]
- 41. Srivastava S, Saxena GN, Keshwani P, Gupta R. Comparing the efficacy and safety profile of sitagliptin versus glimepiride in patients of type 2 diabetes mellitus inadequately controlled with metformin alone. J Assoc Physicians India 2012; 60: 27–30. [PubMed] [Google Scholar]
- 42. Logue J, Walker JJ, Leese G et al. Association between BMI measured within a year after diagnosis of type 2 diabetes and mortality. Diabetes Care 2013; 36(4): 887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferreira‐González I, Permanyer‐Miralda G, Busse JW et al. Methodologic discussions for using and interpreting composite endpoints are limited, but still identify major concerns. J Clin Epidemiol 2007; 60(7): 651–7. [DOI] [PubMed] [Google Scholar]
- 44. Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials. Greater precision with greater uncertainty? JAMA 2003; 289(19): 2554–9. [DOI] [PubMed] [Google Scholar]
- 45. Freemantle N, Calvert M. Composite outcomes. Final comment for now..3. J Clin Epidemiol 2007; 60(7): 662. [Google Scholar]
- 46. Einarson TR, Garg M, Kaur V, Hemels ME. Composite endpoints in trials of type‐2 diabetes. Diabetes Obes Metab 2014; 16(6): 492–499. [DOI] [PubMed] [Google Scholar]
- 47. Fleming GA. Counterpoint–the end point: less is more. J Diabetes Sci Technol 2011; 5(5): 1290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kessler KM. Combining composite endpoints: counterintuitive or a mathematical impossibility? Circulation 2003; 107(9): e70. [DOI] [PubMed] [Google Scholar]
- 49. McIntosh B, Cameron C, Singh SR et al. Second‐line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed‐treatment comparison meta‐analysis. Open Med 2011; 5(1): e35–48. [PMC free article] [PubMed] [Google Scholar]
- 50. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363(9403): 157–63. [DOI] [PubMed] [Google Scholar]
- 51. Lenz M, Richter T, Muhlhauser I. The morbidity and mortality associated with overweight and obesity in adulthood: a systematic review. Dtsch Arztebl Int 2009; 106(40): 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carnethon MR, de Chavez PJ, Biggs ML et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012; 308(6): 581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jerant A, Franks P. Body mass index, diabetes, hypertension, and short‐term mortality: a population‐based observational study, 2000‐2006. J Am Board Fam Med 2012; 25(4): 422–31. [DOI] [PubMed] [Google Scholar]
- 54. Tobias M. Global control of diabetes: information for action. Lancet 2011; 378(9785): 3–4. [DOI] [PubMed] [Google Scholar]
- 55. Doehner W, Erdmann E, Cairns R et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co‐morbidity: an analysis of the PROactive study population. Int J Cardiol 2012; 162(1): 20–6. [DOI] [PubMed] [Google Scholar]
- 56. Tobias DK, Pan A, Jackson CL et al. Body‐mass index and mortality among adults with incident type 2 diabetes. N Engl J Med 2014; 370(3): 233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thomas G, Khunti K, Curcin V et al. Obesity paradox in people newly diagnosed with type 2 diabetes with and without prior cardiovascular disease. Diabetes Obes Metab 2014; 16(4): 317–325. [DOI] [PubMed] [Google Scholar]
- 58. Vina J, Borras C, Gomez‐Cabrera MC. Overweight, obesity, and all‐cause mortality. JAMA 2013; 309(16): 1679. [DOI] [PubMed] [Google Scholar]
- 59. Doehner W. Overweight, obesity, and all‐cause mortality. JAMA 2013; 309(16): 1679–80. [DOI] [PubMed] [Google Scholar]
- 60. Willett WC, Hu FB, Thun M. Overweight, obesity, and all‐cause mortality. JAMA 2013; 309(16): 1681. [DOI] [PubMed] [Google Scholar]
- 61. Grandy S, Fox KM, Bazata DD. Health‐related quality of life association with weight change in type 2 diabetes mellitus: perception vs. reality. Int J Clin Pract 2013; 67(5): 455–61. [DOI] [PubMed] [Google Scholar]
- 62. Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med 2008; 25(3): 245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Choudhary P, Amiel SA. Hypoglycaemia: current management and controversies. Postgrad Med J 2011; 87(1026): 298–306. [DOI] [PubMed] [Google Scholar]
- 64. Ismail‐Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011; 154(8): 554–9. [DOI] [PubMed] [Google Scholar]
- 65. Alvarez GF, Tofe PS, Krishnarajah G, Lyu R, Mavros P, Yin D. Hypoglycaemic symptoms, treatment satisfaction, adherence and their associations with glycaemic goal in patients with type 2 diabetes mellitus: findings from the Real‐Life Effectiveness and Care Patterns of Diabetes Management (RECAP‐DM) Study. Diabetes Obes Metab 2008; 10(Suppl. 1): 25–32. [DOI] [PubMed] [Google Scholar]
- 66. Bron M, Marynchenko M, Yang H, Yu AP, Wu EQ. Hypoglycemia, treatment discontinuation, and costs in patients with type 2 diabetes mellitus on oral antidiabetic drugs. Postgrad Med 2012; 124(1): 124–32. [DOI] [PubMed] [Google Scholar]
- 67. Bohannon NJ. Individualized treatment of type 2 diabetes mellitus using noninsulin agents: clinical considerations for the primary care physician. Postgrad Med 2012; 124(4): 95–108. [DOI] [PubMed] [Google Scholar]
- 68. Bennett WL, Balfe LM, Faysal JM. AHRQ's comparative effectiveness research on oral medications for type 2 diabetes: a summary of the key findings. J Manag Care Pharm 2012; 18(1 Suppl. A): 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Veterans Affairs DoD . Clinical practice guideline for the management of the diabetes mellitus, 2010.
- 70. International Diabetes Federation (IDF) . Clinical Guidelines Task Force Global Guideline for Type 2 Diabetes. Brussels: IDF Communications, 2012. [Google Scholar]
- 71. American Diabetes Association . Standards of Medical Care in Diabetes – 2014. Diabetes Care 2014; 37(Suppl. 1): S14–80. [DOI] [PubMed] [Google Scholar]
- 72. del Prato PS, LaSalle J, Matthaei S, Bailey CJ. Tailoring treatment to the individual in type 2 diabetes practical guidance from the Global Partnership for Effective Diabetes Management. Int J Clin Pract 2010; 64(3): 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]


