Figure 1.
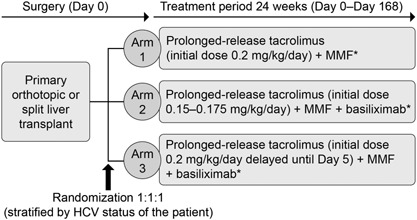
DIAMOND study design. Multicenter, randomized, open‐label, parallel‐group comparative Phase IIIb study. *0 mg–1,000 mg IV bolus corticosteroid (pre‐, intra‐, or post‐operatively) on Day 0. Arm 2 only: if the patient had not received treatment for an acute rejection episode and the last recorded trough level recorded was ≥ 5 ng/mL at Day 43, then the dose was reduced by 20–25%. HCV, hepatitis C virus; IV, intravenous; MMF, mycophenolate mofetil.
