Figure 2.
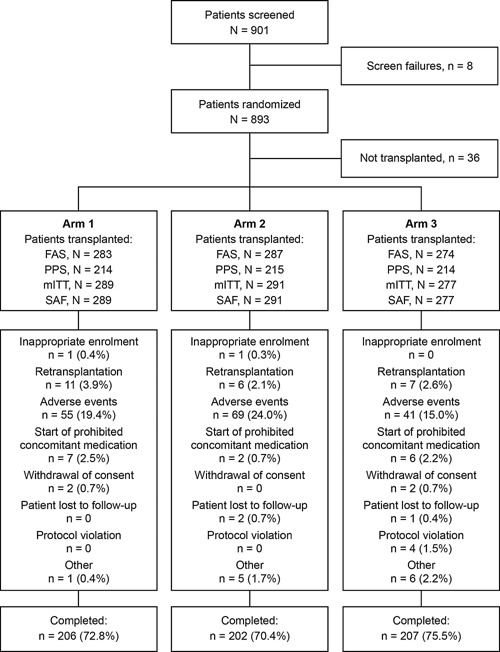
Patient disposition and reasons for discontinuation. Arm 1: Prolonged‐release tacrolimus (initial dose 0.2 mg/kg/day) + MMF; Arm 2: Prolonged‐release tacrolimus (initial dose 0.15–0.175 mg/kg/day) + MMF + basiliximab; Arm 3: Prolonged‐release tacrolimus (initial dose 0.2 mg/kg/day delayed until Day 5) + MMF + basiliximab. Four patients in Arm 3 had protocol violations (FAS): interruption of study medication > 7 consecutive days (two patients), SAE (one patient), and received mycophenolic acid (one patient); AEs were given as the primary reason for discontinuation by the study investigators. The types of AEs leading to discontinuation were not reported, as patients may have had multiple AEs at the time of discontinuation. AE, adverse event; FAS, full‐analysis set; mITT, modified intent to treat; MMF, mycophenolate mofetil; PPS, per‐protocol set; SAF, safety‐analysis set; SAE, serious adverse event.
