Figure 5.
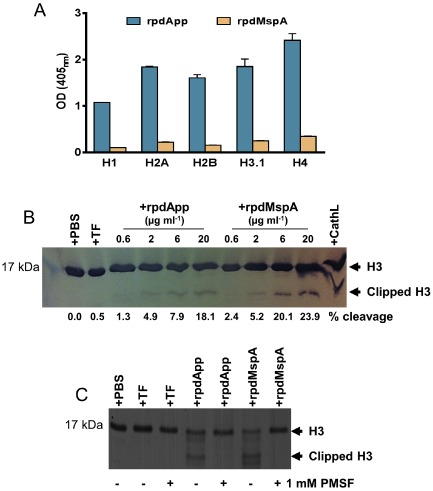
rpdApp or rpdMspA interaction with histones.
A. ELISA plates were coated with recombinant human histones (5 μg ml−1). Following washing, rpdApp and rpdMspA (5 μg ml−1) were added; bound autotransporters were detected using rabbit anti‐TF, followed by anti‐rabbit IgG alkaline phosphatase conjugate. Specific binding was calculated by subtracting the BSA (negative control) values from the histone‐autotransporter absorbance. Tests were performed in triplicate wells and on two independent occasions. Values represent the mean + SD from two experiments.
B. Dose‐dependent cleavage of recombinant histone H3.1 by rpdMspA and rpdApp. Histones were incubated with rpdMspA or rpdApp at 37°C for 15 h. Cathepsin L (CathL; 6 μg ml−1) and TF (20 μg ml−1) were used as positive and negative controls for cleavage, respectively. Reactions were stopped by the addition of SDS‐PAGE sample buffer and cleavage products subjected to SDS‐PAGE. Proteins were strained with Simply Blue Safe stain (Invitrogen). The percentage of clipped H3.1 shown for each lane was determined by densitometry using ImageJ software for data acquisition.
C. Cleavage of histone H3.1 by rpdMspA and rpdApp could be inhibited by 1 mM PMSF, confirming that the serine endopeptidase activity of rpdApp and rpdMspA was responsible for cleavage.
