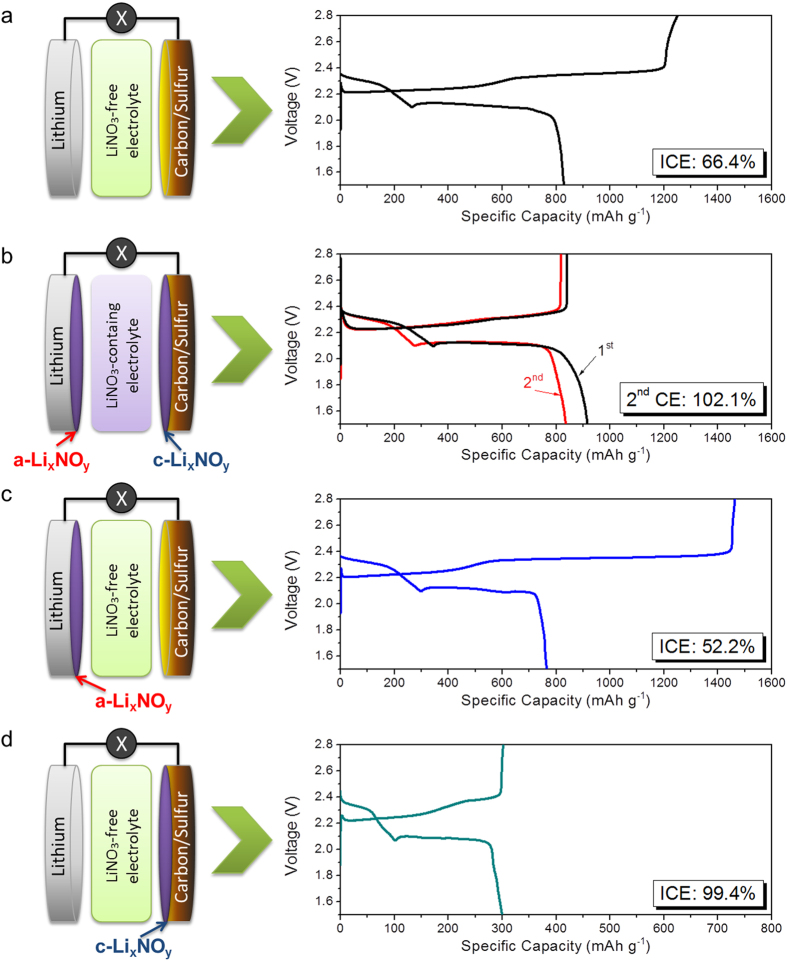
Figure 2. Galvanostatic discharge-charge voltage profiles of Li-S batteries (with sulfur-graphite composite).
(a) 1st cycle of a Li-S cell cycled in LiNO3-free electrolyte, with an ICE of 66.4% as the benchmark. (b) The 2 cycles of the cell cycled in LiNO3-contained electrolyte which was later dissembled to obtained LixNOy coated Li anode and carbon/sulfur cathode. The reduction of LiNO3 on cathode leads to a CE value of ~100%. LixNOy passivation layers formed on both sides of electrodes, assigned to a-LixNOy for anode side and c-LixNOy for cathode side, respectively. (c) The first cycle of the reassembled cell from the used Li anode (with a-LixNOy surface coating) and a fresh carbon/sulfur cathode in LiNO3-free electrolyte, showing an ICE of 52.2%. (d) The first cycle of the reassembled cell with a fresh Li anode and the used carbon/sulfur cathode (with c-LixNOy surface coating) in LiNO3-free electrolyte, giving an ICE of 99.4%.