Figure 1. Electrophoresis profile and trypsin inhibitory activity of aqueous and ethanolic extracts from Momordica charantia.
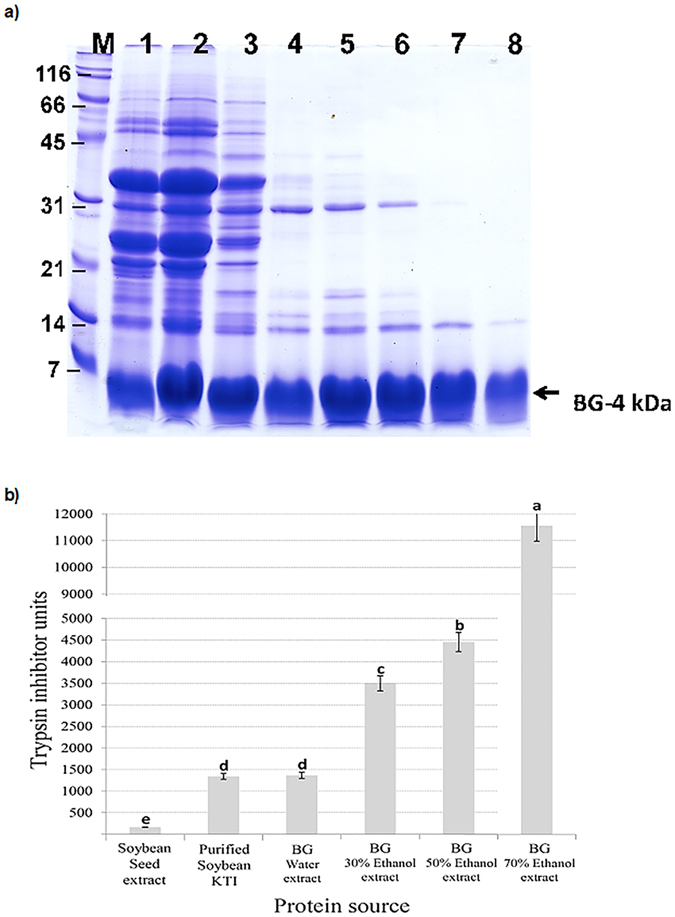
(a) SDS-PAGE profile of different Momordica charantia extracts indicates that increasing ethanol concentrations in the extraction medium led to isolation and purification of a novel peptide termed BG-4. (b) Trypsin inhibitory activity of Momordica charantia extracts is associated with the degree of BG-4 purity. Increasing BG-4 purity led to increased capability of the extract to inhibit trypsin, the purified BG-4 peptide from 70% ethanol extraction is 8.6 times more effective in inhibiting trypsin as compared to purified soybean Kunitz trypsin inhibitor. Mean values represented as bars with different letter(s) are significantly different from each other (P < 0.05, n > 3).
