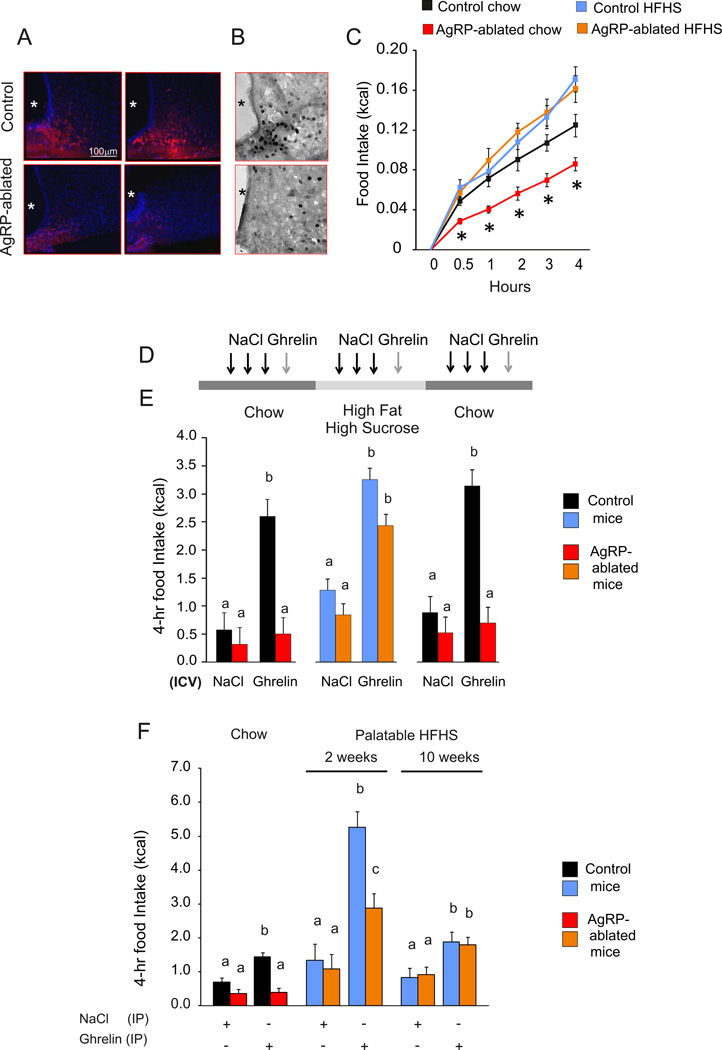
Figure 1. Food palatability restores ghrelin-induced feeding in AgRP-ablated mice.
(A and B) Representative confocal images of brain sections after injection (IV) of active fluorescent ghrelin (A) and immunohistochemistry for c-fos after 24 h fast (B) of AgRP-ablated (lower panel) and control (top panel) mice. Blue: Hoechst, red: incorporated fluorescent ghrelin (A), dark; Fos immune-staining (B). *:3rd ventricle. (C) 4-h caloric response to fast in AgRP-ablated (red, orange) or control (black, blue) mice presented with chow (black, red) or HFHS diet (blue, orange). (D) Representative time bar of three (daily) ICV injections of vehicle, and octanoyled ghrelin on the fourth day in control (black, blue) and AgRP-ablated (red, orange) mice presented consecutively with a chow, HFHS diet and then chow diet. Three days of acclimation were allowed with the new diet before the next series of injection. (E) 4-h cumulative caloric intake in 4-month-old control (black, blue) and AgRP-ablated mice (red, orange) after ICV injection of NaCl or octanoyled ghrelin injection. (F) 4-h cumulative caloric intake after IP injection of octanoyled ghrelin in 4-month-old control (black, blue) and AgRP-ablated mice (red, orange) fed with a chow or 2 or 10 weeks of HFHS diet. n=6 minimum in each group. *, indicated P<0.05, control vs AgRP-ablated mice. a, b and c differ significantly (P<0.05). Data are expressed as mean ± SEM. (See also Figure S1).