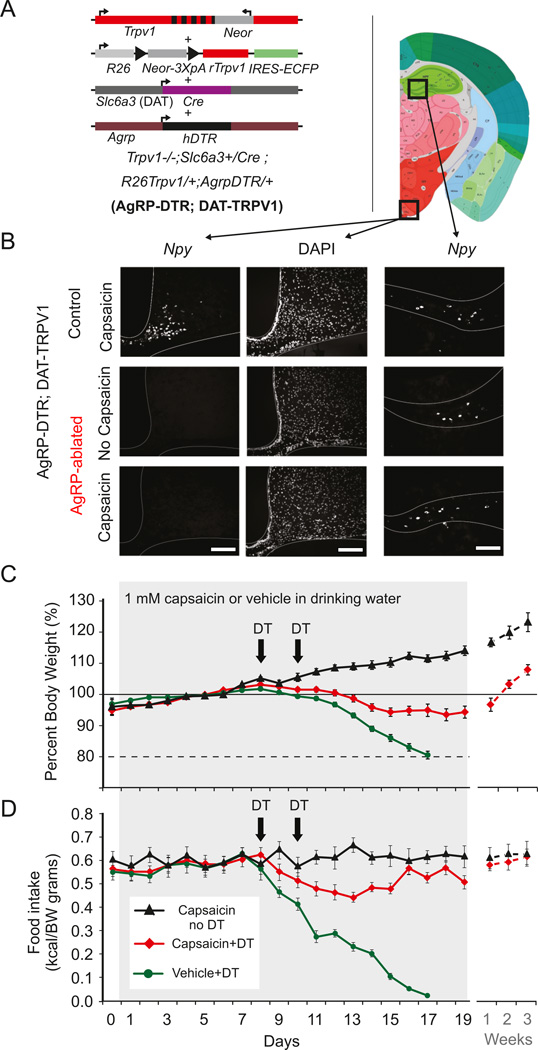
Figure 3. Mild activation of dopamine neurons prevents starvation following acute ablation of AgRP neurons in adult mice.
(A) Trpv1−/−; Slc6a3+/Cre; R26Trpv1/+ mice (DATTRPV1; (Guler et al., 2012)) were crossed to AgrpDTR/+ mice (Luquet et al., 2005) on the C57Bl/6 background to generate AgRPDTR. Resulting triple transgenic mice conditionally express rat Trpv1 (rTrpv1) gene from Gt(ROSA)26Sor (R26) locus only in dopamine neuron expressing CRE recombinase under the dopamine transporter (Slc6a3 or DAT) promoter on the Trpv1 knockout background (Trpv1−/−; Neor: neomycin resistance gene; IREs-ECFP: internal ribosome entry site-enhanced cyan fluorescent protein). (B) Fluorescent in situ hybridization for NPY (left and right panel) and DAPI counterstaining (middle panel) in arcuate (left panel) and hippocampus (right panel) of control (upper panel) and AgRPDTR mice (lower panel) injected with diphtheria toxin. Scale bar, 200 µm. (C) Body weight change (%) and food intake (kcal/g BW) of AgRPDTR; DATTRPV1 with capsaicin (1 mM) in drinking water (black triangle; n=6 mice), or injected twice with diphtheria toxin (DT) with capsaicin (red diamond; n=14 mice) or vehicle (green circle; n=15 mice) in drinking water. Following AgRP-neuron ablation, all of the AgRPDTR; DATTRPV1 mice with no access to capsaicin treatment had a concomitant decrease in food intake and body weight within 7 days. Mice at 80% of baseline bodyweight were not rescued and sacrificed.