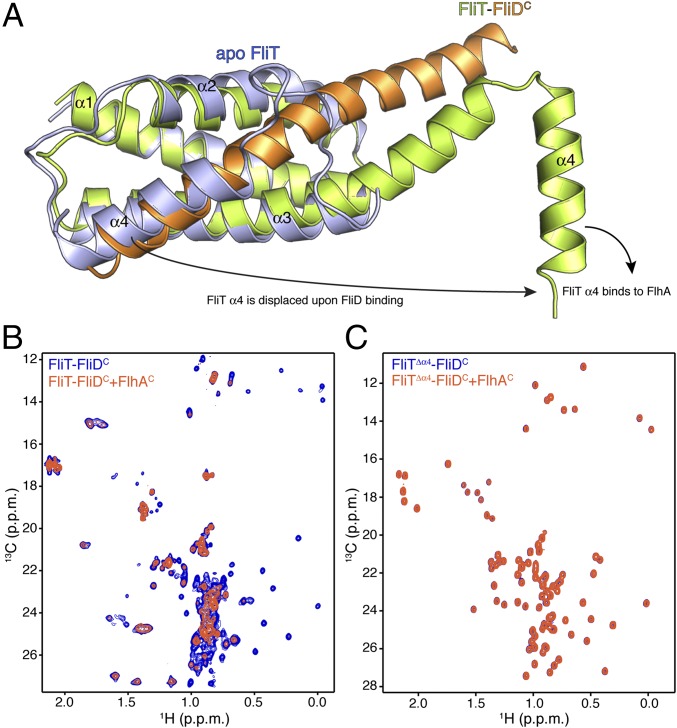
Fig. 4.
FliD binding releases FliT helix α4 and activates the complex for FlhA binding. (A) Superposition of free FliT on the FliT−FliDC complex structure. The FliT helix α4, which our NMR data show is released upon FliD binding, is modeled in the structure. (B) 1H-13C heteronuclear multiple quantum coherence (HMQC) spectra of FliT−FliDC (blue) and in the presence of FlhAC (orange). The data show that FliT−FliDC binds to FlhAC and that the interaction is mediated primarily by FliT helix α4. (C) 1H-13C HMQC spectra of FliTΔα4−FliDC (blue) and in the presence of FlhAC (orange). The data show that truncation of FliT helix α4 abrogates binding to FlhAC.