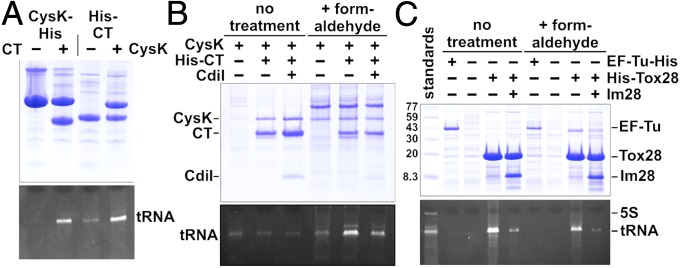
Fig. 6.
tRNA binding to Ntox28 nuclease domains. (A) Cross-linking of tRNA to CysK/CdiA-CTEC536 complexes. E. coli cell lysates containing CysK and/or CdiA-CT(H178A)EC536 were treated with formaldehyde, and cross-linked nucleoprotein complexes were purified by Ni2+-affinity chromatography. Purified samples were analyzed by SDS/PAGE (Top) and 50% urea PAGE (Bottom) to visualize proteins and nucleic acid, respectively. (B) CdiIEC536 blocks tRNA cross-linking to CysK/CdiA-CTEC536. E. coli cell lysates containing CysK-His6, CysK-His6/CdiA-CT(H178A)EC536, and CysK-His6/CdiA-CT(H178A)/CdiIEC536 were treated with formaldehyde where indicated, followed by Ni2+-affinity chromatography, and gel analysis as described in A. (C) Tox28Rlac interacts stably with tRNA substrate. Cell lysates containing His6-Tox28(H114A)Rlac and ImmRlac were treated as described above. To ascertain cross-linking specificity, EF-Tu-His6 was also purified and analyzed for tRNA binding.