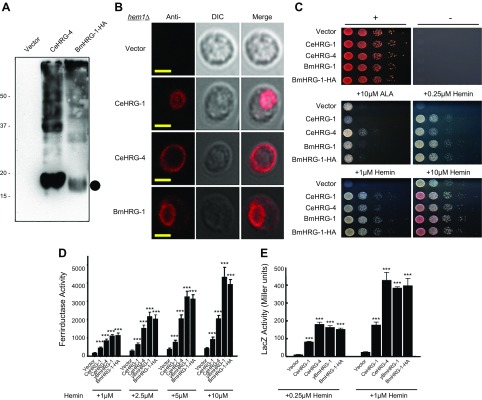
Figure 2.
BmHRG-1 overexpression, localization, and functionality in S. cerevisiae. A) Immunoblot analysis of yeast transformants overexpressing C-terminal HA-tagged versions of CeHRG-4 (5 μg total extract) or BmHRG-1 (30 μg total extract) in the hem1Δ strain. The predicted molecular mass (∼17 kDa) of BmHRG-1 is indicated by the filled circle. B) Indirect immunofluorescence images of the hem1Δ strain transformed with vector alone, CeHRG-1-HA, CeHRG-4-HA, or BmHRG-1-HA. Scale bars, 5 μm. C) Spot-growth assay. Yeast (hem1Δ) transformed with empty vector, CeHRG-4, CeHRG-1, BmHRG-1, or BmHRG-1-HA spotted in serial dilutions on plates supplemented with 10 μM ALA or the indicated hemin concentration and incubated at 30°C for 3 d before imaging. Positive control (top left): +0.4% glucose, +250 μM ALA; negative control (top right): −ALA, −hemin. D) The hem1Δfre1Δfre2ΔMET3-FRE1 strain was transformed with empty vector, CeHRG-1, CeHRG-4, BmHRG-1, or BmHRG-1-HA and ferric reductase activity (nanomoles/106cells/min) was measured in the presence of hemin (as indicated). E) The hem1Δ strain stably expressing either the pYes-DEST52 vector alone, CeHRG-1, CeHRG-4, or BmHRG-1 (untagged as well as HA tagged) were transformed with pCYC1-LacZ. Following growth in the indicated concentrations of hemin after 12 h, β-galactosidase activity of the cell lysates was determined. The results are means ± sem of 3 independent experiments. ***P < 0.001, Student’s t test.