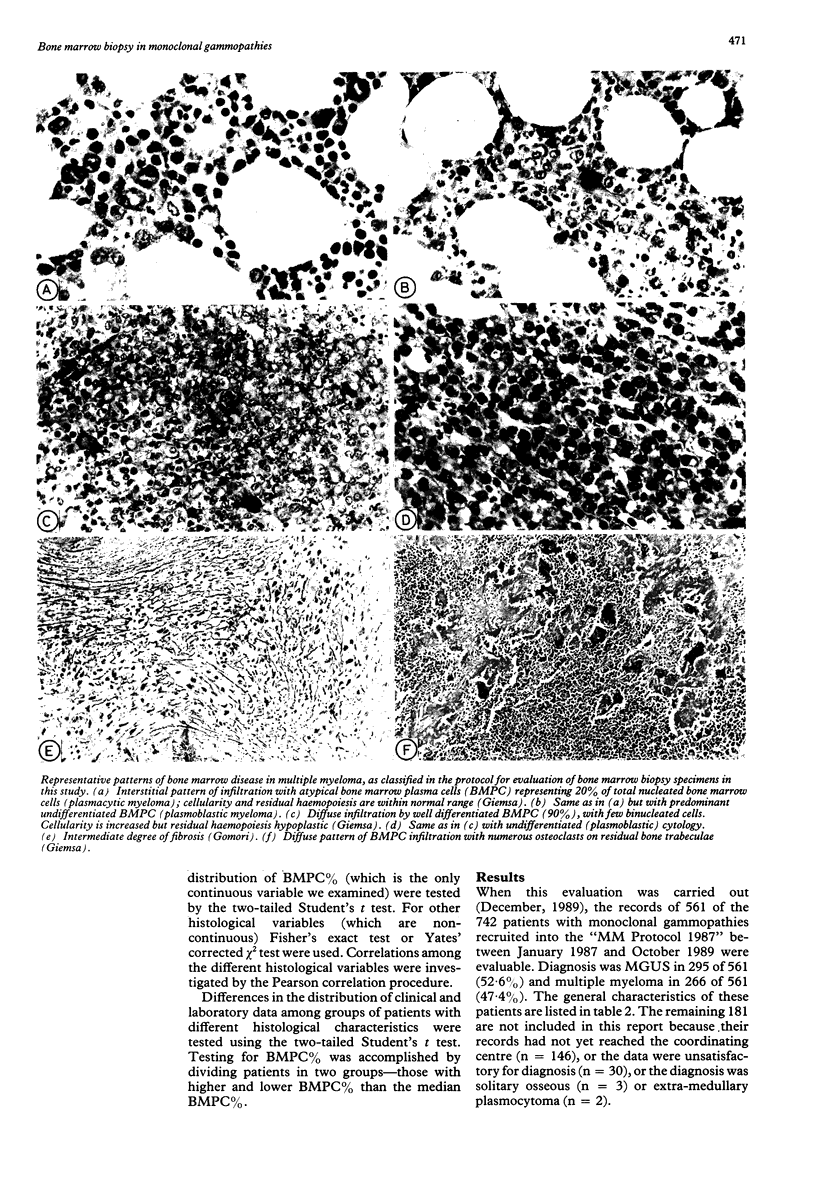
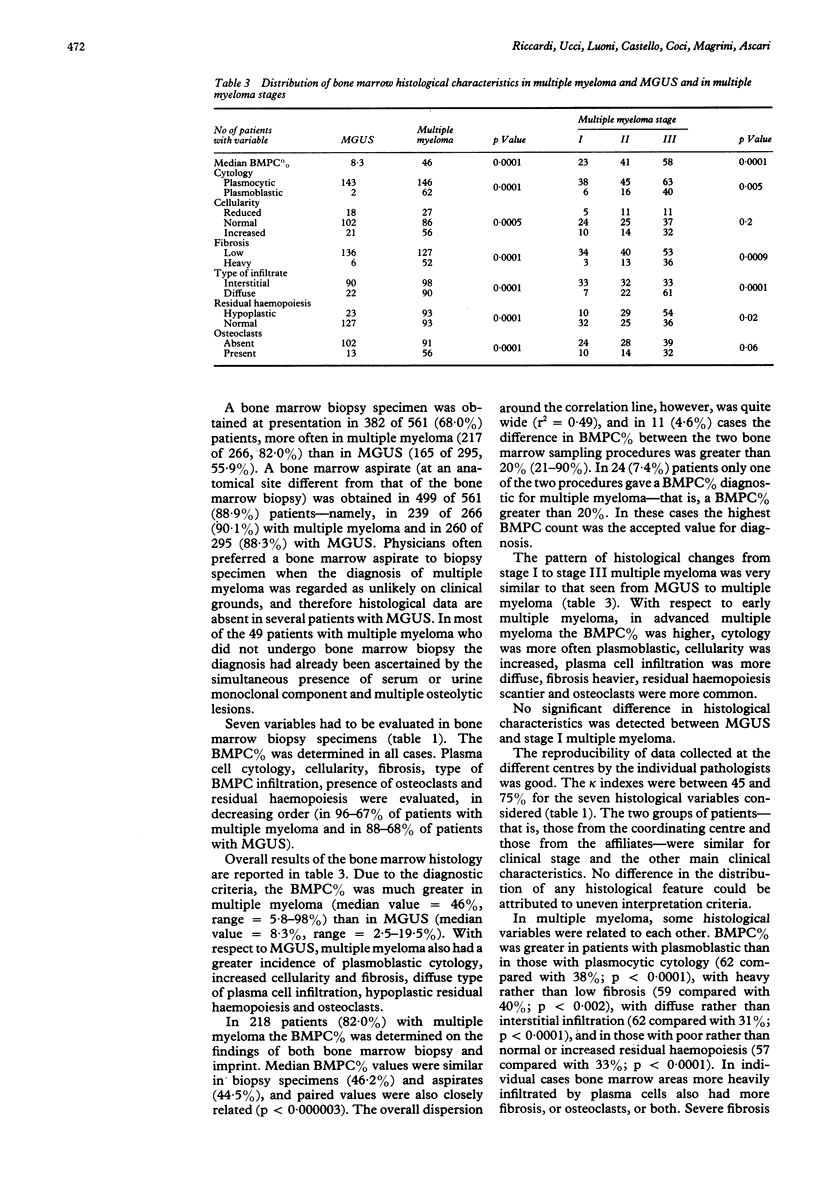
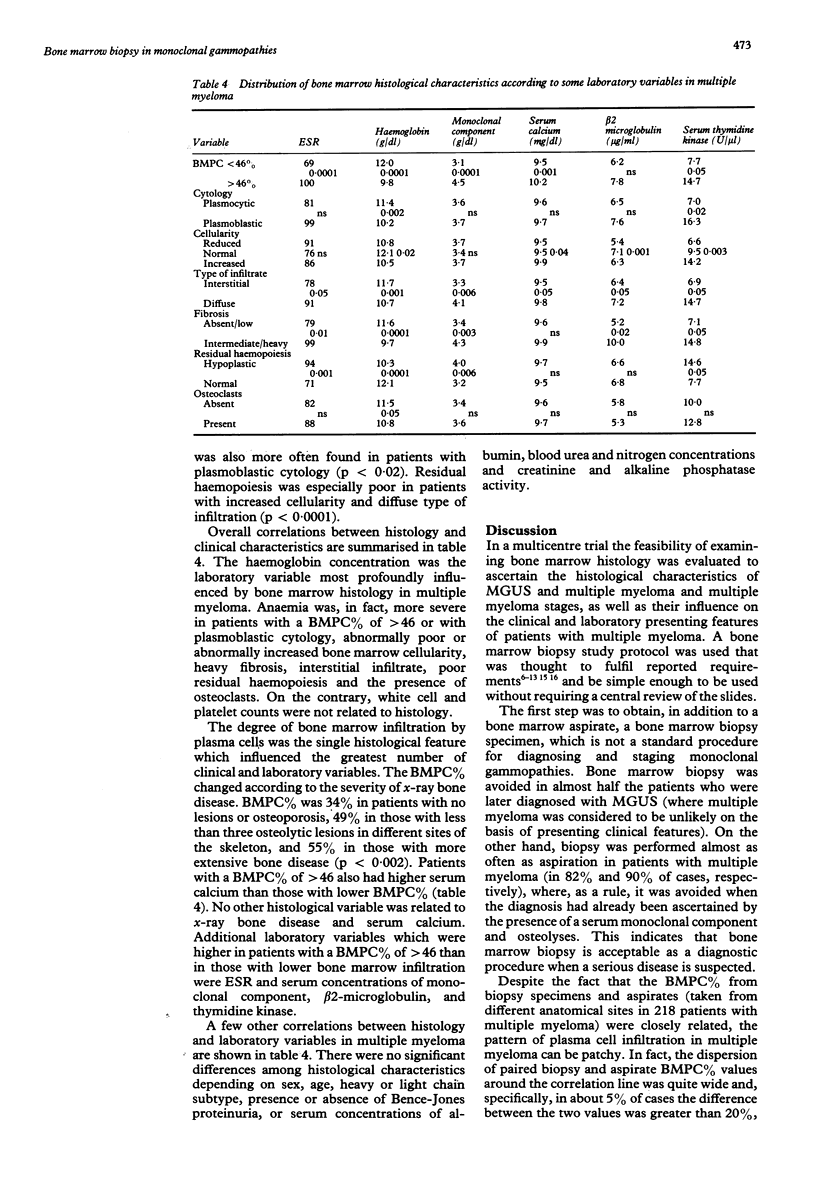
Abstract
Between January 1987 and October 1989, 561 consecutive untreated patients with monoclonal gammopathy of undetermined clinical importance (MGUS) (n = 295) or with multiple myeloma (n = 266) were evaluated in a multicentre trial. Both bone marrow biopsy and aspiration (performed at different anatomical sites) were required at presentation. Bone marrow biopsy data indicated that changes in bone marrow composition from MGUS to early multiple myeloma and to advanced multiple myeloma followed a precise pattern, including an increased percentage of bone marrow plasma cells (BMPC%), a shift from plasmocytic to plasmoblastic cytology, an increase in bone marrow cellularity and fibrosis, a change in bone marrow infiltration (becoming diffuse rather than interstitial), a decrease in residual haemopoiesis and an increase in osteoclasts. In multiple myeloma the BMPC% of biopsy specimens and aspirate were closely related, although in 5% of cases the difference between the two values was greater than 20%. Some histological features were remarkably associated with each other. For example, BMPC% was higher in cases with plasmoblastic cytology, heavy fibrosis, or reduced residual haemopoiesis. Anaemia was the clinical characteristic most influenced by bone marrow histology. The BMPC% was the only histological variable which affected the greatest number of clinical and laboratory characteristics, including, besides haemoglobin concentration, erythrocyte sedimentation rate, radiographic skeletal bone disease, and serum concentrations of monoclonal component, calcium, beta 2-microglobulin and thymidine kinase activity. These data indicate that comparative bone marrow histology in monoclonal gammopathies has clinical importance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartl R., Frisch B., Burkhardt R., Fateh-Moghadam A., Mahl G., Gierster P., Sund M., Kettner G. Bone marrow histology in myeloma: its importance in diagnosis, prognosis, classification and staging. Br J Haematol. 1982 Jul;51(3):361–375. doi: 10.1111/j.1365-2141.1982.tb02791.x. [DOI] [PubMed] [Google Scholar]
- Bartl R., Frisch B., Fateh-Moghadam A., Kettner G., Jaeger K., Sommerfeld W. Histologic classification and staging of multiple myeloma. A retrospective and prospective study of 674 cases. Am J Clin Pathol. 1987 Mar;87(3):342–355. doi: 10.1093/ajcp/87.3.342. [DOI] [PubMed] [Google Scholar]
- Bartl R. Histologic classification and staging of multiple myeloma. Hematol Oncol. 1988 Apr-Jun;6(2):107–113. doi: 10.1002/hon.2900060209. [DOI] [PubMed] [Google Scholar]
- Bataille R., Chappard D., Alexandre C., Dessauw P., Sany J. Importance of quantitative histology of bone changes in monoclonal gammopathy. Br J Cancer. 1986 Jun;53(6):805–810. doi: 10.1038/bjc.1986.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille R., Grenier J. Serum beta 2 microglobulin in multiple myeloma. A critical review. Eur J Cancer Clin Oncol. 1987 Dec;23(12):1829–1832. doi: 10.1016/0277-5379(87)90047-2. [DOI] [PubMed] [Google Scholar]
- Bauermeister D. E. Quantitation of bone marrow reticulin--a normal range. Am J Clin Pathol. 1971 Jul;56(1):24–31. doi: 10.1093/ajcp/56.1.24. [DOI] [PubMed] [Google Scholar]
- Carter A., Hocherman I., Linn S., Cohen Y., Tatarsky I. Prognostic significance of plasma cell morphology in multiple myeloma. Cancer. 1987 Sep 1;60(5):1060–1065. doi: 10.1002/1097-0142(19870901)60:5<1060::aid-cncr2820600522>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975 Sep;36(3):842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fritz E., Ludwig H., Kundi M. Prognostic relevance of cellular morphology in multiple myeloma. Blood. 1984 May;63(5):1072–1079. [PubMed] [Google Scholar]
- Garrett I. R., Durie B. G., Nedwin G. E., Gillespie A., Bringman T., Sabatini M., Bertolini D. R., Mundy G. R. Production of lymphotoxin, a bone-resorbing cytokine, by cultured human myeloma cells. N Engl J Med. 1987 Aug 27;317(9):526–532. doi: 10.1056/NEJM198708273170902. [DOI] [PubMed] [Google Scholar]
- Greipp P. R., Raymond N. M., Kyle R. A., O'Fallon W. M. Multiple myeloma: significance of plasmablastic subtype in morphological classification. Blood. 1985 Feb;65(2):305–310. [PubMed] [Google Scholar]
- Krzyzaniak R. L., Buss D. H., Cooper M. R., Wells H. B. Marrow fibrosis and multiple myeloma. Am J Clin Pathol. 1988 Jan;89(1):63–68. doi: 10.1093/ajcp/89.1.63. [DOI] [PubMed] [Google Scholar]
- Kyle R. A. Multiple myeloma: review of 869 cases. Mayo Clin Proc. 1975 Jan;50(1):29–40. [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- Merlini G., Waldenström J. G., Jayakar S. D. A new improved clinical staging system for multiple myeloma based on analysis of 123 treated patients. Blood. 1980 Jun;55(6):1011–1019. [PubMed] [Google Scholar]
- Mundy G. R., Ibbotson K. J., D'Souza S. M., Simpson E. L., Jacobs J. W., Martin T. J. The hypercalcemia of cancer. Clinical implications and pathogenic mechanisms. N Engl J Med. 1984 Jun 28;310(26):1718–1727. doi: 10.1056/NEJM198406283102607. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Raisz L. G., Cooper R. A., Schechter G. P., Salmon S. E. Evidence for the secretion of an osteoclast stimulating factor in myeloma. N Engl J Med. 1974 Nov 14;291(20):1041–1046. doi: 10.1056/NEJM197411142912001. [DOI] [PubMed] [Google Scholar]
- Ralston S. H. The pathogenesis of humoral hypercalcaemia of malignancy. Lancet. 1987 Dec 19;2(8573):1443–1446. doi: 10.1016/s0140-6736(87)91139-1. [DOI] [PubMed] [Google Scholar]
- Salmon S. E. Immunoglobulin synthesis and tumor kinetics of multiple myeloma. Semin Hematol. 1973 Apr;10(2):135–144. [PubMed] [Google Scholar]
- Simonsson B., Källander C. F., Brenning G., Killander A., Ahre A., Gronowitz J. S. Evaluation of serum deoxythymidine kinase as a marker in multiple myeloma. Br J Haematol. 1985 Oct;61(2):215–224. doi: 10.1111/j.1365-2141.1985.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Stepán J. J., Neuwirtová R., Pacovský V., Formánková J., Silinková-Málková E. Biochemical assessment of bone disease in multiple myeloma. Clin Chim Acta. 1984 Sep 29;142(2):203–209. doi: 10.1016/s0009-8981(84)80001-7. [DOI] [PubMed] [Google Scholar]
- Ucci G., Riccardi A., Luoni R., Spriano P., Merlini G., Danova M., Cassano E., Molinari E., Ascari E. Serum thymidine kinase and beta-2 microglobulin in monoclonal gammopathies. Tumori. 1987 Oct 31;73(5):445–449. doi: 10.1177/030089168707300503. [DOI] [PubMed] [Google Scholar]
- Valentin-Opran A., Charhon S. A., Meunier P. J., Edouard C. M., Arlot M. E. Quantitative histology of myeloma-induced bone changes. Br J Haematol. 1982 Dec;52(4):601–610. doi: 10.1111/j.1365-2141.1982.tb03936.x. [DOI] [PubMed] [Google Scholar]
- Vandermolen L., Rice L., Lynch E. C. Plasma cell dyscrasia with marrow fibrosis. Clinicopathologic syndrome. Am J Med. 1985 Sep;79(3):297–302. doi: 10.1016/0002-9343(85)90307-9. [DOI] [PubMed] [Google Scholar]