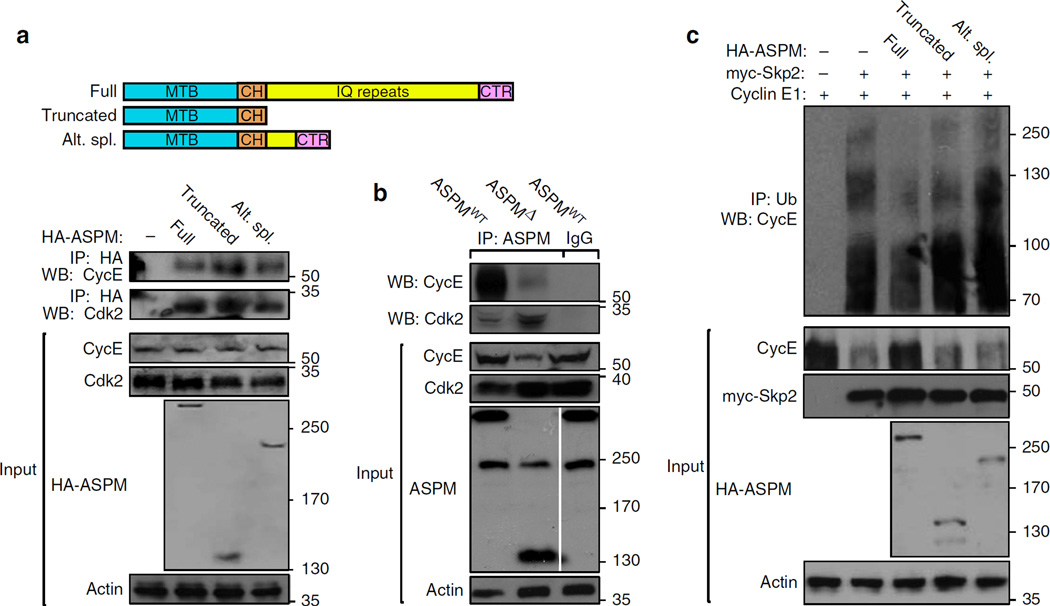
Figure 9. ASPM isoforms interaction with Cyclin E and regulation of its ubiquitination.
(a) Schematic representation of full length, truncated and alternatively spliced ASPM cDNAs used for the co-IP studies. Plasmids expressing the indicated HA-tagged human ASPM cDNAs were co-transfected with Cyclin E1 into HEK293T cells. After 36 h cells were treated with MG132 for further 12 h and lysed. Lysates were IPed with α-HA and immunoblotted with α-Cyclin E Abs. MTB, microtubule-binding domain; CH, calponin binding domain; IQ, calmodulin binding domain; CTR, C-terminus. (b) co-IP analyses of protein extracts from 5 × E11 WT and ASPMΔ cortices. Extracts, IPed with α-ASPM Abs, were immunoblotted with Cyclin E and Cdk2 Abs, demonstrating a direct and physiological evidence of endogenous ASPM:Cdk2/Cyclin E association. Data further illustrate the association of endogenous truncated and alternatively spliced ASPM with the Cdk2/Cyclin E complex, as shown in Fig. 6a. Note in the input blots, full-length (345 kDa) ASPM proteins were not detected in mutant embryos, whereas the alternate splice variant (205 kDa) expression remained intact. Instead, a truncated (155 kDa) ASPM product was noticed in mutant embryos. (c) Plasmids expressing the indicated HA-tagged human ASPM cDNAs were co-transfected with Cyclin E1 and myc-Skp2 into HEK293T cells. After 36 h cells were treated with MG132 for further 12 h and lysed. Lysates were IPed with α-ubiquitin and immunoblotted with α-Cyclin E Abs.