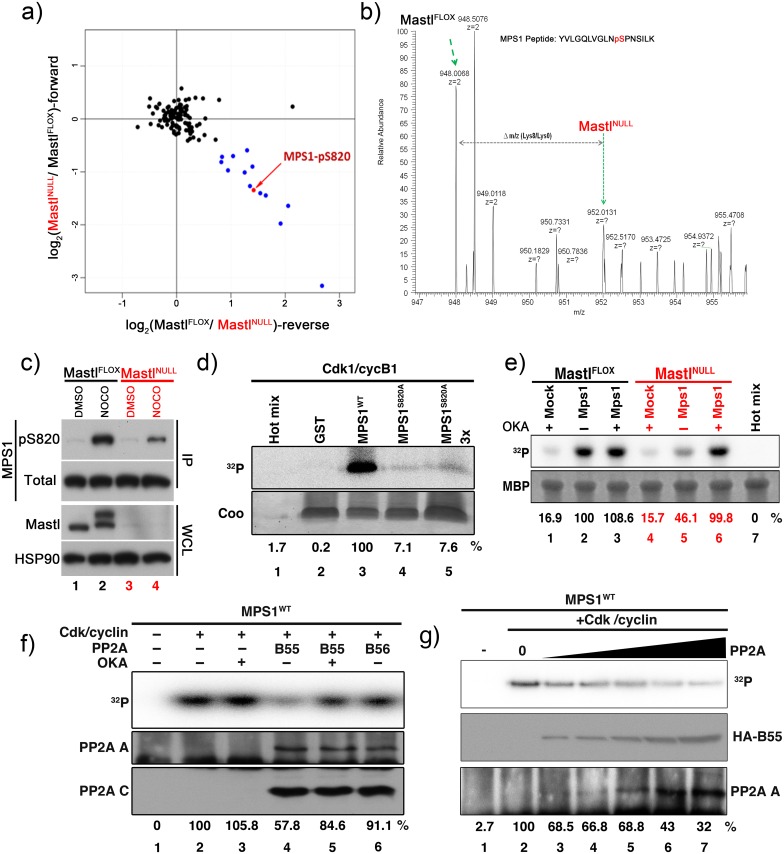
Fig 3. Mastl is required for phosphorylation and full activity of MPS1.
(A) Phospho-proteomic analysis of mitotic arrested MEFs was performed using SILAC. The log2 SILAC ratio of phospho-peptides in each mass spectrometry run is plotted as the forward experiment against the reverse experiment. Data points colored in blue denote down-regulated phosphorylation sites in MastlNULL cells with at least 1.5 fold change. Black points indicate peptides with unchanged phosphorylation status. (B) MPS1 S820 phospho-peptide MS spectrum: the intensities of SILAC peptide pairs (heavy signal corresponding to MastlNULL cells and light signal to MastlFLOX) are shown. (C) Immortalized MEFs expressing lentivirally transduced HA-tagged MPS1 (human) were synchronized and released as indicated in Fig 2D. Cells were treated with or without 500ng/ml nocodazole between hours 24–28 after release. Whole cell lysate (WCL) for each condition were prepared and subjected to immunoblot analysis for Mastl and HSP90. HA-MPS1 was immunoprecipitated (IP) using anti-HA antibodies and probed for phosphorylated or total MPS1. (D, F, G) GST-tagged recombinant wild type (WT) or non-phosphorylatable mutant (S820A) MPS1 peptide fusion protein was purified and subjected to in vitro kinase assay with recombinant Cdk1/cyclin B1 complexes (D; see Methods; MPS1S820A 3x designated an amount of three times of the substrates from lane 4). For F and G, phosphorylated GST-MPS1WT peptide fusion protein was subjected to in vitro phosphatase assay using immunoprecipitated HA-tagged B55 or B56 complexes in presence (+) or absence (-) of 200nM OKA. A representative image for each experiment is shown and representative results for quantification of 32P intensity signals of phosphorylated GST-MPS1 peptide fusion protein are shown as percentage derived from lane 6 for D, lane 2 for F-G. For F-G, levels of the PP2A A scaffold and PP2A C catalytic subunits co-immunoprecipitated are shown. For G, in vitro phosphatase assay was performed using immunoprecipitated PP2A complexes from increasing amounts of whole cell lysates from HA-B55 overexpressing 293T cells (25, 50, 100, 250 and 500μg) and phosphorylated GST-MPS1 peptide fusing protein as a substrate. Western blots for HA-B55 (middle panel) and the PP2A A scaffold subunit (bottom panel) are shown. (E) Immortalized MEFs synchronously released into full growth medium were transfected with plasmids encoding Myc-tagged MPS1 (human). Cells were arrested in mitosis by treating with 500ng/ml nocodazole for 4 hours between hours 24–28 after serum release. Cells were additionally treated with 200nM OKA (+) or DMSO (-) between hours 27–28 as indicated. Myc-MPS1 was immunoprecipitated using anti-Myc antibodies and subjected to in vitro kinase assay using 5μg MBP as a substrate and recombinant Cdk1/cyclin B1 as kinase (see Methods). A representative experiment is shown and representative results for quantification of 32P intensity signals of phosphorylated MBP are shown as percentage derived from lane 2.