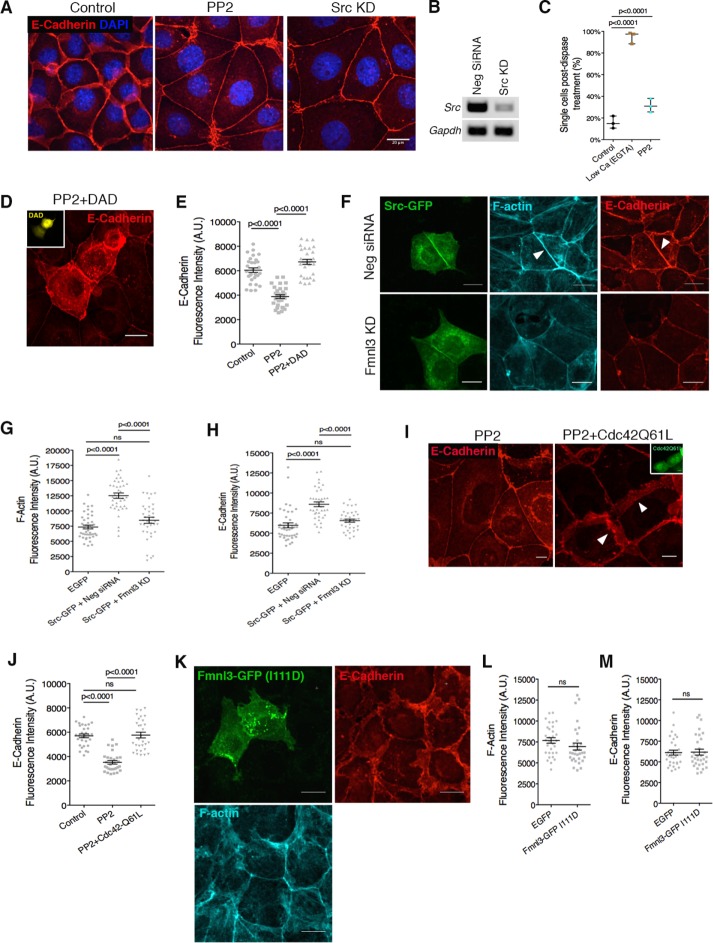
FIGURE 5:
Src kinase and Cdc42 are upstream activators of formin activity at the AJ. (A) Src kinase inhibition via PP2 treatment or siRNA-mediated KD phenocopies mDia1 or Fmnl3 KD. (B) PCR analysis for efficiency of Src KD. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as control. (C) Dispase assay for PP2-treated monolayers. Three independent experiments, with mean ± SD. (D) Activation of endogenous mDia1 via DAD expression (mVenus fluorescence in inset) rescues the Src-inhibition phenotype. Note the dramatic augmentation of the AJ in transfected cells in comparison to nontransfected neighbors. (E) Quantification of E-cadherin fluorescence intensity at the junction for D. Control monolayers were transfected with a GFP vector; ≥30 junctions from transfected cells for each condition from three experiments, with mean ± SEM. (F) Full-length Src-GFP localizes to the AJ, resulting in elevated levels of F-actin and E-cadherin (arrowheads, top). Src-GFP expression combined with Fmnl3 KD abrogates junctional F-actin and E-cadherin augmentation (bottom). (G, H) Quantification of F-actin and E-cadherin fluorescence intensities for F; ≥35 junctions from transfected cells for each condition from three experiments, with mean ± SEM. (I) Expression of constitutively active Cdc42 (GFP shown in inset) rescues the Src-inhibition phenotype. Note the restoration of lateral junctions (white arrowheads) in transfected cells vs. nontransfected neighbors. (J) Quantification of E-cadherin fluorescence intensity for I. Control monolayers were transfected with a GFP vector; ≥ 28 junctions from transfected cells for each condition from three experiments, with mean ± SEM. (K) Fmnl3-GFP (I111D) does not localize to AJ, with no effect on F-actin or E-cadherin. (L, M) Quantification of F-actin and E-cadherin fluorescence intensities for K; ≥26 junctions from transfected cells per condition from three experiments, with mean ± SEM. Statistical significance assessed using one-way ANOVA in C, E, G, H, and J; Student’s t test in L and M. Scale bars, 20 μm (A, D, F, and K), 10 μm (I).