Fig 2.
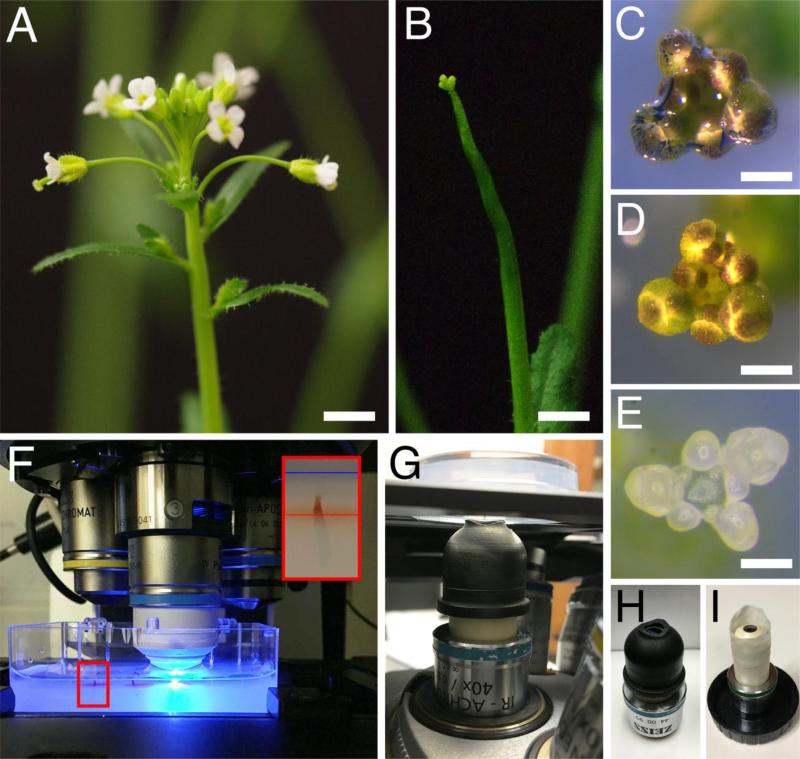
Preparation of the shoot apices for imaging. (A-E) Dissection of a shoot apex for imaging. Inflorescence before (A) and after (B) removal of siliques and older flowers. (C-D) Shoot apex immersed in water in the imaging dish, with an air bubble trapped at the tip (C) and after removal of the air bubble (D). (E) Shoot apex in the imaging dish, after dissection of flower buds older than stage 5. (F-G) View of shoot apices in the imaging dish, on the stage of an upright (F) and inverted (G) confocal microscopes. In (F), a 40x dipping lens is positioned above one of the apices, with the tip of the lens immersed in water. In (G), the shoot apex is positioned upside down above the 40x dipping lens, with a water column connecting the imaging medium to the tip of the lens. The smaller panel in (F) shows a higher magnification view of the area in the red rectangle, with a shoot apex inserted in imaging medium; red and blue lines indicate the surface of the medium and the water, respectively. (H-I) Examples of custom-made devices that allow adding more water at the tip of the dipping lens: a rubber sleeve (H), and a makeshift sleeve made from the finger of a powderless latex glove (G). Bars = 0.5 cm in (A) and (B), 0.1 cm in (C) and (D), and 100 μm in (E).