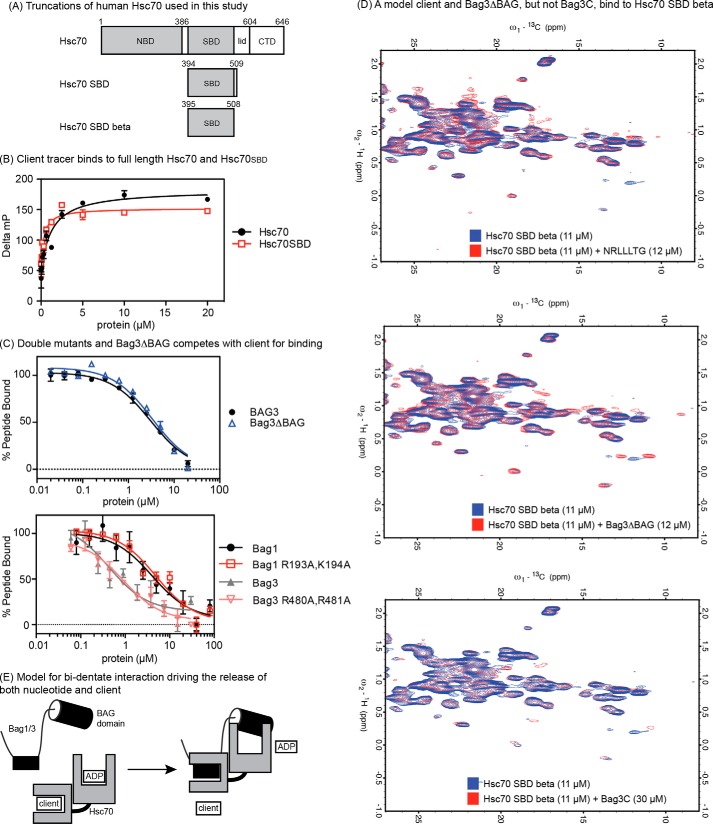
FIGURE 4.
The non-canonical interaction between Bag3 and Hsp72 occurs in the SBD. A, truncations of Hsc70 used in this study. B, client tracer interacts with both full-length Hsc70 and Hsc70SBD. C, Bag1, 3, double mutants and Bag3ΔBAG compete with client for binding to the Hsc70SBD. Results are the average of experiments performed in triplicate. Error is S.E. D, 600 MHz 13C-1H HSQC spectra of 11 μm 13C 15N Hsc70 (395–508) showing binding to a model client (NRLLLTG), to Bag3ΔBAG and to Bag3C. In each overlay, the apo-protein spectrum is in blue, and the spectrum of the Hsc70 SBDβ domain in the presence of ligand is shown in red. The model client and Bag3ΔBAG cause chemical shift perturbations and appearances of new resonances in slow exchange at ∼1:1, whereas Bag3C only causes minor changes at ∼3:1. E, model for the bi-dentate mechanism of human Bag1 and Bag3. The identity and location of the SBD-binding site is not yet clear (see supplemental Fig. S1).