FIGURE 2.
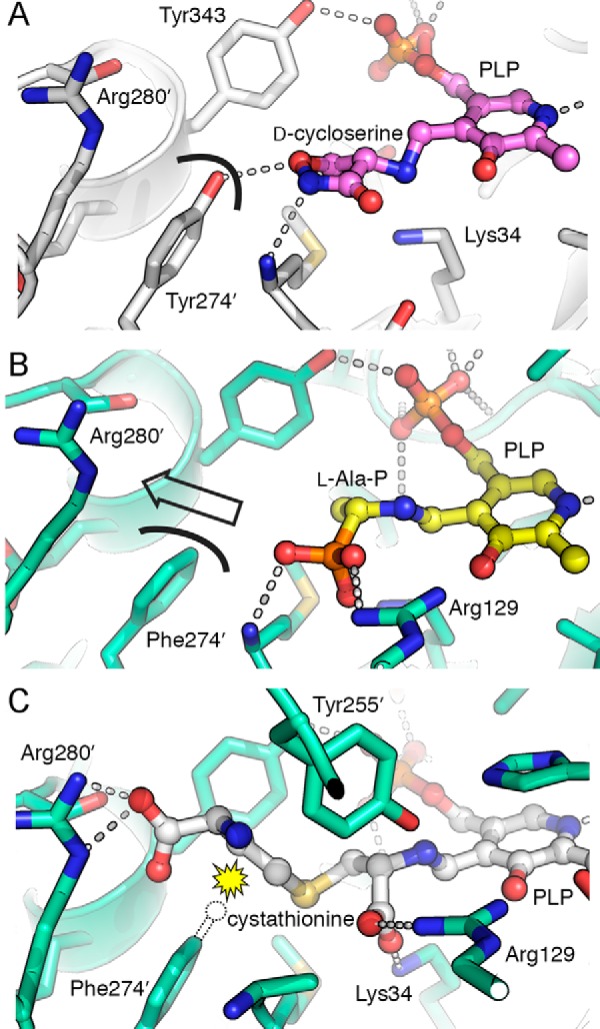
Structural analysis of the Y274F mutation in ALR. A, in the structure of wild type ALR inhibited by d-cycloserine (PDB entry 2RJH) (15), Tyr-274′ is positioned to form a hydrogen bond with the inhibitor. The curved black line shows the surface at the tyrosine hydroxyl group. B, active site of ALR(Y274F) with the l-Ala-P-PLP external aldimine, shown in the same orientation as A. The curved black line represents the surface of Phe-274′. The lack of a hydroxyl group opens a pathway from the substrate toward Arg-280′ (indicated with an arrow). C, modeled position of l-cystathionine in the ALR(Y274F) active site, docked using GOLD. The distal carboxylate of the substrate is perfectly positioned to interact with Arg-280′. A dotted outline indicates the position that the Tyr-274′ hydroxyl group occupies in the wild type ALR structure. The yellow starburst indicates the steric clash that would occur between this hydroxyl group and cystathionine, disfavoring substrate binding. The Y274F mutation removes this steric clash.
