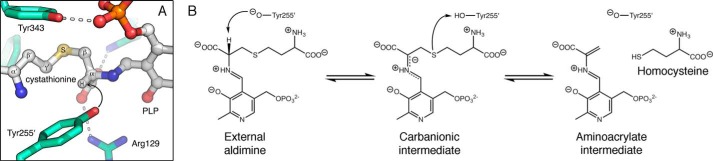
FIGURE 3.
Tyr-255′ is positioned to play a catalytic role in cystathionine β-elimination by ALR(Y274F). A, when l-cystathionine-PLP is docked into the active site using GOLD, the side chain oxygen atom of Tyr-255′ is ∼2.6 Å from the proton that it is poised to abstract from the substrate (black arrow). B, tentative reaction scheme for ALR(Y274F)-catalyzed cystathionine β-elimination. Tyr-255′ has an unusually low pKa (20), so it is assumed to be in the phenolate form for proton abstraction (22). Unlike in CBL, the pyridine nitrogen is not protonated, because of the proximity of Arg-209 in the ALR active site. This results in a resonance-stabilized carbanionic intermediate of unusually high energy (45). By analogy with the CBL mechanism (35), we predict that Tyr-255′ reorients to protonate the leaving group (homocysteine). This would yield the PLP derivative of aminoacrylate, which is the substrate for reverse transaldimination (35), to form iminopropionate and to regenerate the Lys-34-PLP Schiff base. The nature of the carbanionic intermediate and inefficient proton transfer from Tyr-255′ to the leaving group are likely to explain the very poor turnover observed for ALR(Y274F).