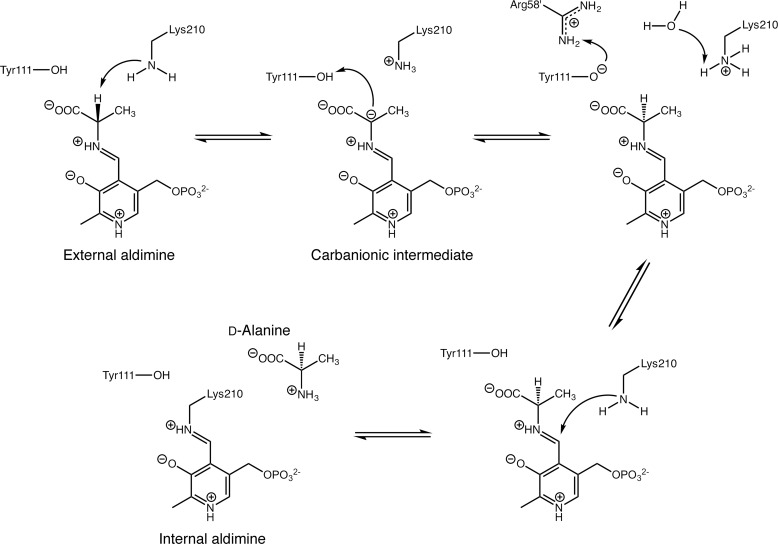
FIGURE 5.
Reaction scheme for racemization of l-alanine, catalyzed by CBL(P113S). Estimates from PROPKA suggest that Tyr-111 is likely to be protonated when the l-Ala-PLP external aldimine is formed. When CBL catalyzes cystathionine β-elimination, the carbanionic intermediate is stabilized as the ketamine quinonoid (36). Here, we show instead the resonance structure of the carbanionic intermediate that is consistent with the ALR mechanism (22). Also, as done previously (22), we have shown the proton exchanges at Tyr-111 (involving Arg-58′) and Lys-210 occurring from the same intermediate, but this is not a necessity. Reverse transaldimination to regenerate the Lys-210-PLP Schiff base is assumed to occur as described for the cystathionine β-elimination reaction.