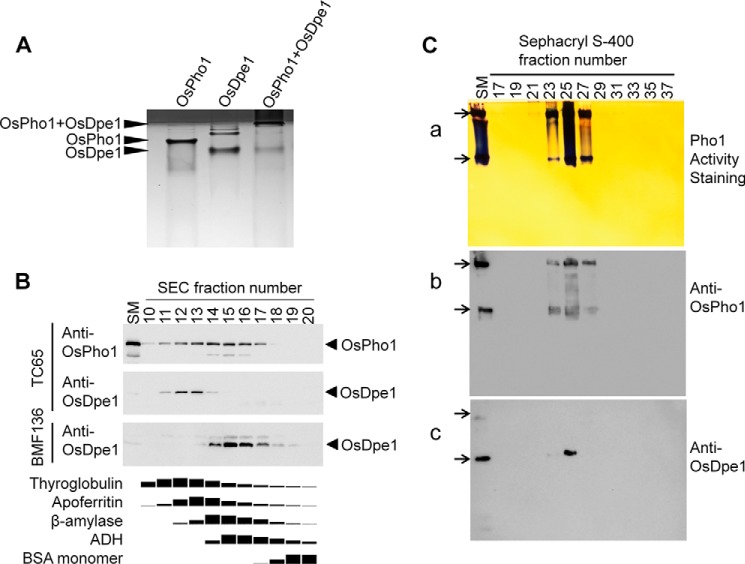
FIGURE 4.
Interaction of OsPho1 and OsDpe1. A, mobility patterns of OsPho1, OsDpe1, and a mixture of OsPho1 and OsDpe1. Native polyacrylamide gel (10%) electrophoresis was performed in Tris/Tricine buffer, and the CBB staining result shows stacking (dark) and separation (bright) gels. B, immunoblot analysis of seed proteins in fractions (1 ml) resolved by Superdex-200 SEC chromatography. 10 μl of each fraction were subjected to 12% SDS-PAGE and subsequently analyzed by immunoblotting using anti-OsPho1 and anti-OsDpe1. The bottom panel depicts the elution profiles of the molecular mass standard proteins (669 kDa, thyroglobulin; 443 kDa, apoferritin; 200 kDa, β-amylase; 150 kDa, alcohol dehydrogenase; 66 kDa, BSA) under the same chromatography conditions. Amounts of the marker proteins in the fractions were determined by using the image analysis software Image J 1.48v after scanning the CBB-stained SDS-PAGE gel. C, co-migration of OsPho1 and OsDpe1. The Sephacryl S-400 fractions were separated on a 7.5% Tris/glycine non-denaturing polyacrylamide gel containing 0.8% glycogen. Activity staining and immunoblot analysis methods are described “Experimental Procedures.” Arrows indicate two OsPho1 activity bands (a) on the native gel and their corresponding locations on the immunoblot membranes of OsPho1 (b) and OsDpe1 (c). SM denotes starting material containing 15 μg of soluble proteins from immature rice seeds (see “Experimental Procedures”).