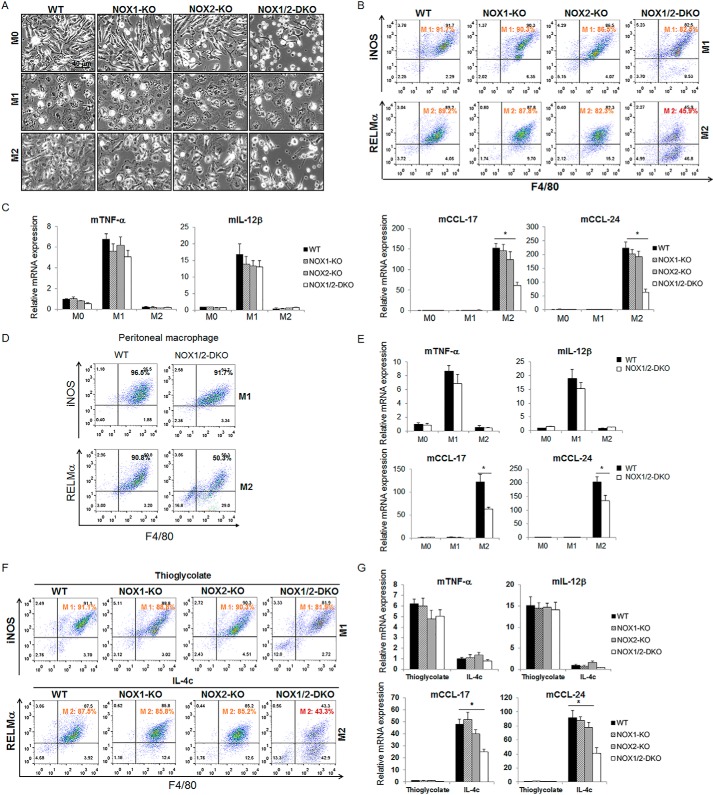
FIGURE 2.
Absence of both NOX1 and NOX2 affects monocyte-to-macrophage differentiation. A, BMMs from C57BL/6 WT, NOX1-KO, NOX2-KO, and NOX1/2-DKO mice were treated with M-CSF (20 ng/ml) for 6 days (M0). On day 6, the M0 cells were treated with LPS (100 ng/ml) and INF-γ (20 ng/ml) for M1 or IL-4 (20 ng/ml) for M2 for 24 h. Representative light microscopy image from three independent experiments are shown. B, cells from A were analyzed by flow cytometry with antibodies to iNOS and F4/80 (M1 population) and to RELMα and F4/80 (M2 population). C, detection of M1 cytokines (TNF-α, ΙL-12β) and M2 chemokines (CCL17, CCL24) by real-time PCR of cells from A. D and E, mouse peritoneal macrophages from WT and NOX1/2-DKO were isolated and cultured overnight. Then macrophages were polarized to M1 or M2 macrophages. Cells were co-stained with the indicated antibodies and analyzed by flow cytometry (D). The expression levels of M1 cytokines (TNF-α, IL-12β) or M2 chemokines (CCL17, CCL24) were detected by real-time PCR (E). F and G, WT, NOX1-KO, NOX2-KO, and NOX1/2-DKO mice (n = 3/group) were injected i.p. with either TG or IL-4c. On day 4 after injection, macrophages were isolated by peritoneal lavage, co-stained with the indicated antibodies, and analyzed by flow cytometry (F). The expression levels of M1 cytokines (TNF-α, IL-12β) or M2 chemokines (CCL17, CCL24) were detected by real-time PCR (G). All results represent the mean ± S.D. from three independent experiments. *, p < 0.05 versus WT.