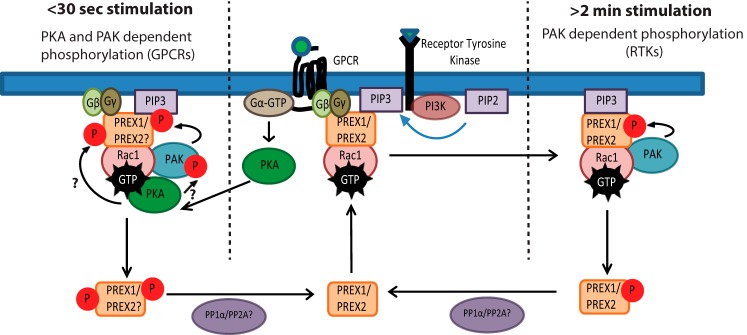
FIGURE 5.
Model for PAK-dependent regulation of PREX1 phosphorylation and function by membrane receptors. PREX phosphorylation can be regulated by either RTKs or GPCRs. After RTK activation of PREX GEF activity, the Rac-dependent PAKs stimulate the phosphorylation of PREX proteins around 1–2 min after treatment with various RTK ligands. This causes a reduction in PIP3 binding and a reduction in PIP3-stimulated GEF activity and potentially weakens the membrane binding of PREX proteins. Downstream of certain GPCRs that are known to activate PKA, PAKs mediate the phosphorylation of PREX1 within 30 s of treatment with an agonist. PREX proteins are activated by Gβγ and PIP3 to promote GTP loading on Rac, which can then activate PAK-dependent phosphorylation of PREX1 or PREX2. Additionally, PKA is activated by Gα, and it then potentially binds to Rac-GTP to phosphorylate and activate PAKs and increase the PAK signal. This would allow for PAK-mediated phosphorylation of PREX proteins that relies on both PAK and PKA. While in this signaling complex, PKA may also phosphorylate PREX, and both PAK and PKA-mediated phosphorylation could provide negative feedback by reducing the binding of PREX to Gβγ and PIP3. PP1α and PP2A dephosphorylation may complete the circuit and allow for PREX proteins to become activated by future stimulation of the receptor.