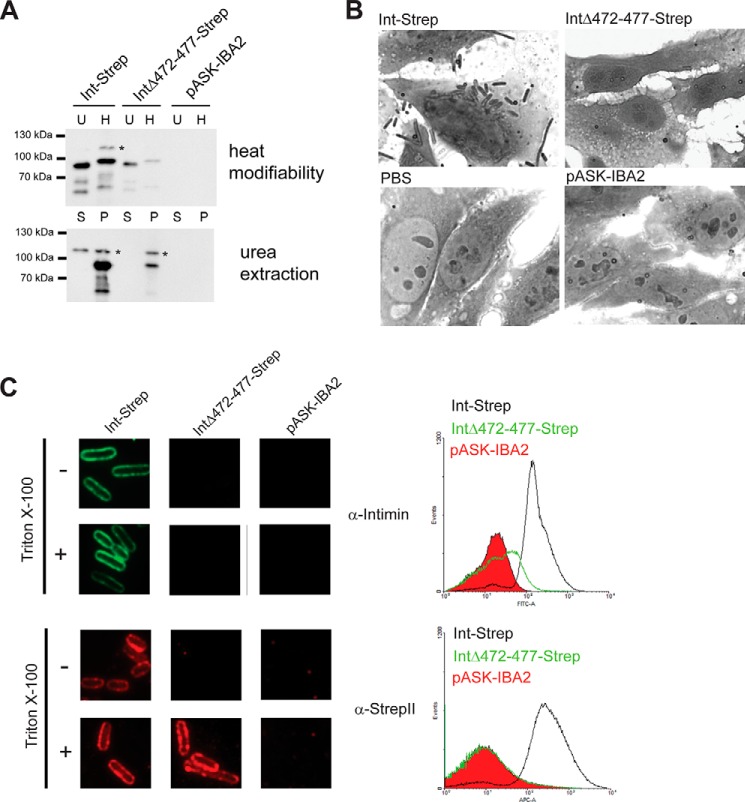
FIGURE 6.
Deletion of a β-strand in intimin D00 leads stalling of passenger secretion. A, membrane insertion and folding of intimin Δ472–477. Western blots using an anti-intimin antibody showing heat modifiability (above) and urea extraction (below) of WT Int-Strep and IntΔ472–477-Strep. Bacteria with the empty vector (pASK-IBA2) were the negative control. For heat modifiability assays, the outer membrane sample was split in half, and one duplicate (H) was heated for 10 min at 95 °C before loading. The other, unheated duplicate (U) was kept at room temperature until loaded. In urea extraction experiments, outer membrane samples were treated with 6 m urea for 1 h and then pelleted at 150,000 × g. Samples were loaded from the supernatant (S) and resuspended pellet (P). Asterisks denote the occasionally observed intimin band at ∼115 kDa of uncertain provenance. B, adhesion of bacteria expressing Int-Strep and IntΔ472–477-Strep to Tir-primed HeLa cells. Bacteria containing the empty vector (pASK-IBA2) and a sample with no bacteria (PBS) served as negative controls. C, surface exposure of IntΔ472–477. Surface exposure of the intimin passenger was assayed both by immunofluorescence microscopy (left) and flow cytometry (right). In both, the protein was detected with both an anti-intimin antibody (upper panels) and an anti-StrepII tag antibody (lower panels). In immunofluorescence microscopy, surface-exposed epitopes are detected both with and without treatment with the detergent Triton X-100, but periplasmically located epitopes can only be detected when the cells are permeabilized with the detergent. In flow cytometry, the histogram for Int-Strep is given in black, the histogram for IntΔ472–477-Strep is in green, and the histogram for the vector control (pASK-IBA2) filled in with red.