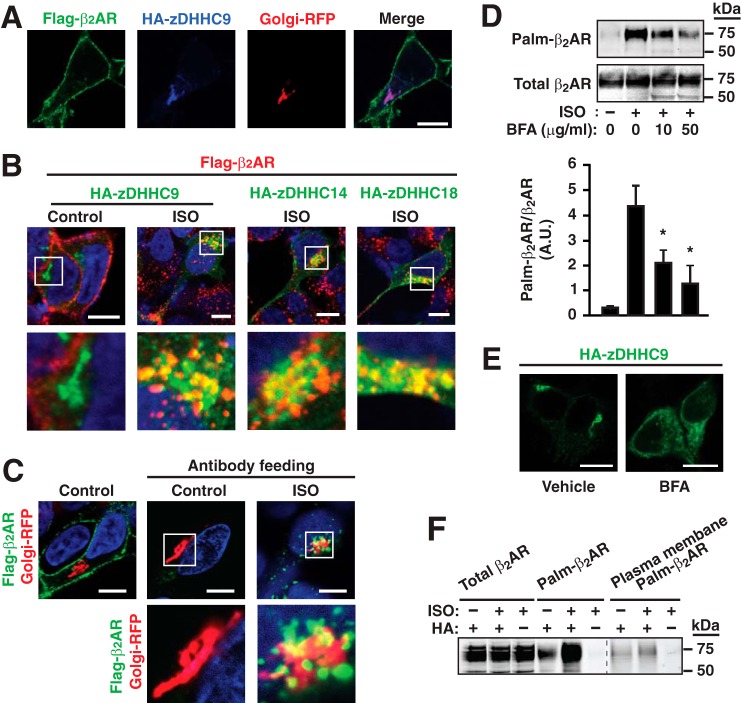
FIGURE 5.
S-Palmitoylation of β2AR Cys-265 is associated with a previously undescribed intracellular itinerary. A, confocal immunofluorescence microscopic analysis in HEK293 cells stably expressing FLAG-tagged wild-type β2AR (green) and transiently transfected with HA-tagged zDHHC9 (blue) as well as the Golgi complex marker Golgi-RFP (red) under basal conditions. β2AR is localized predominantly to the plasma membrane, whereas HA-zDHHC9 is localized to the Golgi complex. B, in HEK293 cells stably expressing FLAG-β2AR (red), transiently transfected with HA-tagged zDHHC9, -14, or -18 (green), and stimulated with ISO (10 μm, 1 h), β2AR are present at a perinuclear location (consistent with localization to the Golgi complex) where they co-localize with zDHHC9, -14, or -18. Merged images are shown at a relatively low and a higher magnification in the top (white boxes) and bottom rows, respectively. Scale bar = 10 μm. C, in HEK293 cells stably expressing FLAG-tagged wild-type β2AR (green) and Golgi RFP, cell-surface FLAG-β2AR was labeled with anti-FLAG M2 antibody in the absence or presence of 10 μm ISO for 30 min, and surface antibody was removed by acid wash. Hoechst 33342 was employed for nuclear staining (blue). This antibody feeding assay reveals directly that a population of β2AR traffics from the plasma membrane to the Golgi complex upon ISO stimulation. D, treatment with brefeldin A (BFA) (50 μg/ml, 2 h) suppresses ISO-induced S-palmitoylation of β2AR Cys-265 in a dose-dependent fashion. n = 3; *, p < 0.05 versus ISO without brefeldin A by ANOVA. E, treatment with brefeldin A (50 μg/ml, 2 h) results in delocalization of HA-zDHHC9 from a perinuclear concentration to a diffuse cytoplasmic distribution. Scale bar = 10 μm. F, cell-surface biotin labeling followed by acyl-RAC and then pulldown of biotinylated and S-palmitoylated proteins with streptavidin followed by Western blotting for the β2AR demonstrates that at least some proportion of β2AR S-palmitoylated at Cys-265 following ISO stimulation (10 μm, 1 h) returns to the plasma membrane following transit from the Golgi complex. Data shown are representative of two separate experiments.