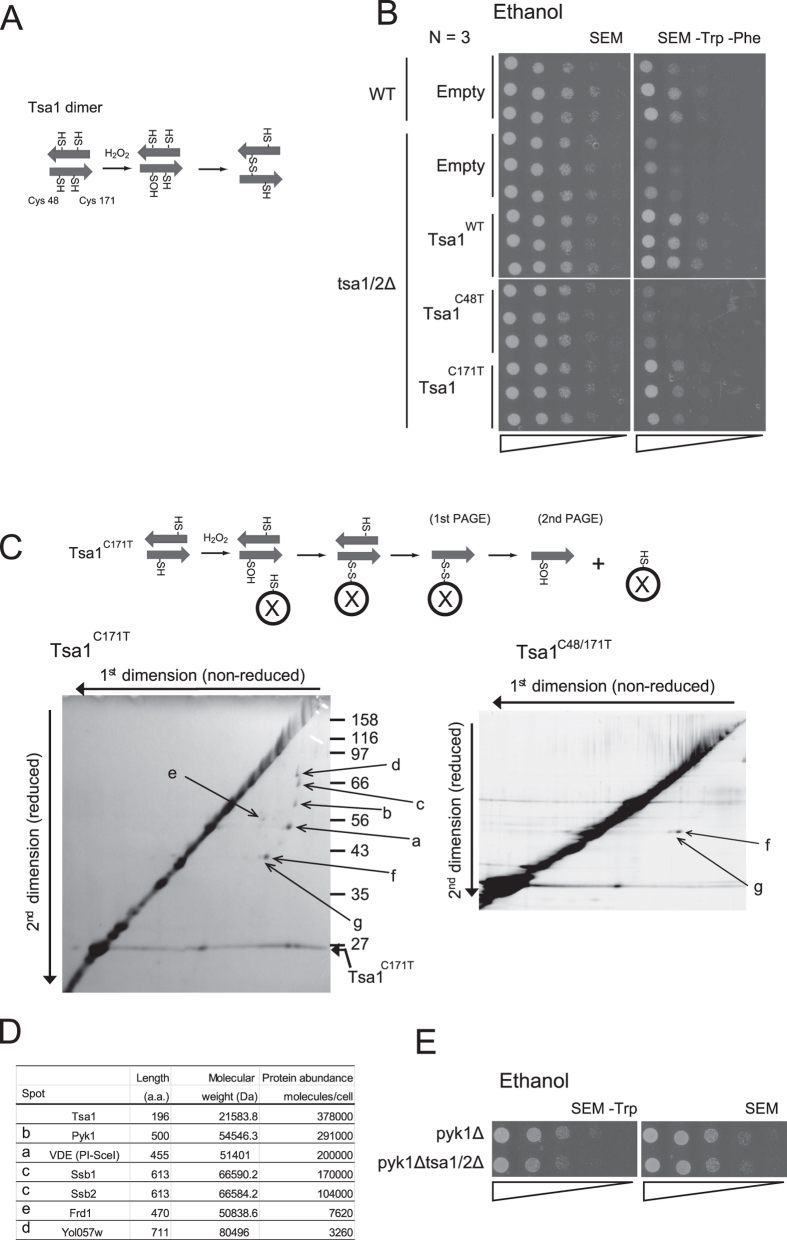
Figure 3. Isolation and identification of Tsa1 binding proteins.
(A) A schematic illustration of the Tsa1 homodimer. In response to H2O2, an intermolecular disulfide bond is formed between an H2O2-induced sulfenic acid from a peroxidatic Cys48 and a resolving Cys171 of the partner (disulfide dimer). (B) Spot assay for tsa1/2∆ cells carrying plasmids (pRS315) for Tsa1WT, Tsa1C171T, and Tsa1C48T on SEM or SEM –Trp –Phe (N = 3). ‘Empty’ indicates cells carrying empty vector, pRS315. For plasmid selection, Leu was also omitted from the medium. ‘WT’ indicates the parental strain BY4742 control cells expressing endogenous Tsa1. (C) H2O2-induced sulfenic acid formation in Cys48 (Cys48-OH) is thought to be stabilized in Tsa1C171T. Thus, putative target proteins (X) that can react with Cys48-SOH may be trapped using HAT-tagged Tsa1C171T. Tsa1C171T binding proteins were purified from yeast cells (BY4742 tsa1Δ) expressing HAT-tagged Tsa1C171T. Cells were grown in SDM –Leu and were treated with 0.5 mM H2O2 for 5 min. Proteins in the purified fraction were fractionated by two-dimensional gel electrophoresis. The first dimension (horizontal) and the second dimension (vertical) were performed under non-reduced and reduced conditions, respectively (left panel). Non-specific proteins that interacted with HAT-tagged Tsa1C48T, C171T mutants were also fractionated (right panel). A gradient gel (10% to 16%) was used for the second dimension. (D) Amino acid length, molecular weight (http://www.yeastgenome.org/), and protein abundance32 for each spot are shown. See also Figure Supplement 2 for the amino acid sequence information. (E) Spot assay of pyk1∆ cells and pyk1∆ tsa1/2∆ cells on SEM –Trp agar plates. Representative data are reported, and the original data (N = 3) are shown in Figure Supplement 1.