Abstract
Background:
Hydroxychloroquine can improve most underlying coronary risk factors; however, there are a few studies on the effects of hydroxychloroquine on blood glucose and insulin resistance. The current study aimed to assess the effects of hydroxychloroquine on blood glucose control status as well as on level of lipid profile and inflammatory biomarkers in prediabetic patients.
Materials and Methods:
In a randomized, double-blinded, controlled trial, 39 consecutive patients who were suffering from prediabetes and were referred to the Isfahan Endocrinology Center in January 2013 were randomly assigned to receive hydroxychloroquine (6.5 mg/kg/day) (n = 20) or placebo (n = 19) for 12 weeks. The biomarker indices and anthropometric parameters were tested before and after completion of treatment.
Results:
In both groups of patients receiving hydroxychloroquine and placebo, except for serum level of insulin that was significantly elevated after treatment by hydroxychloroquine, the changes in other parameters remained insignificant. Both groups experienced increase of insulin level, but this change was considerably higher in those groups receiving hydroxychloroquine. The group receiving hydroxychloroquine experienced reduction of glucose at 60 min of Oral Glucose Tolerance Test (OGTT) test after intervention, while the placebo group experienced increase of blood glucose at the same time.
Conclusion:
The use of hydroxychloroquine may increase the serum insulin level in patients with prediabetic states who are at risk of developing diabetes mellitus.
Keywords: Hydroxychloroquine, insulin resistance, prediabetes condition
INTRODUCTION
Prediabetes is considered as an intermediate clinical condition without complete definitive criteria of diabetes, but with blood glucose higher than the normal range. Patients with a prediabetes state are at increased risk for diabetes mellitus in the future,[1] and are also predisposed to cardiovascular disorders. Furthermore, the higher prevalence of cardiovascular risk factors in these patients can explain the necessity for controlling these factors appropriately to reduce the risk for epidemic cardiovascular disorders.[2,3] Insulin resistance is a common abnormality in prediabetes, leading to increased risk for coronary atherosclerosis. In fact, this abnormality in combination with other classic coronary risk factors as well as chronic subclinical inflammation is responsible for progression of coronary artery disease in these patients. Thus, insulin sensitizing is suggested to reduce the incidence of coronary atherosclerosis via inhibition of inflammatory processes.[3] The main fundament of inflammatory defects in prediabetes patients is inactivation of B cells, which has been reported in 55–58% of affected patients.[4] Hydroxychloroquine is an effective drug in the treatment of inflammatory disorders such as rheumatoid arthritis and systemic lupus erythromatosis. Recent evidences have focused its major role in glucose hemostasis in hyperglycemic patients.[5] Some studies show that treatment with hydroxychloroquine for a period of 6 months can effectively decrease blood glucose and also hemoglobin A1c probably due to increase insulin production and secretion from B cells,[6] or to decrease insulin clearance.[6] In fact, this drug can reduce insulin post receptor clearance and facilitate glucose transfer by insulin.[6] Moreover, hydroxychloroquine can inhibit inflammatory biomarkers as well as regulate the level of lipid profile, leading to a reduced risk of diabetes mellitus.[6,7] Based on the previous studies,[8,9] it seems likely that hydroxychloroquine can improve most underlying coronary risk factors; however, the exact effects of hydroxychloroquine on blood glucose and insulin resistance among the Iranian population has not been discussed yet. The current study aimed to assess the effects of hydroxychloroquine on blood glucose control status as well as lipid profile and inflammatory biomarkers in prediabetic patients.
MATERIALS AND METHODS
Study participants
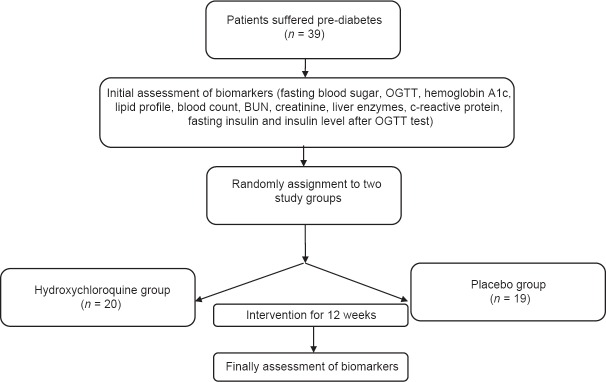
In a randomized, double-blinded, controlled trial, 39 consecutive patients suffering from prediabetes, who were referred to the Isfahan Endocrinology Center in January 2013, were included in the study [Figure 1]. Prediabetes state was defined according to the criteria of the American Diabetes Association (2010). The main inclusion criteria were age >18 years, serum hemoglobin level ≥10 g/dL, white blood cell count ≥4000/mL, plasma platelet count in the range of 150.000–400.000/mL, glomerular filtration rate ≥70 cc/mL, serum Aspartat aminoteransferase (AST) and Alanin Aminoteransferase (ALT levels less than 1.5-times of the upper normal limit, and normal ocular examination within 1 year before the study for ruling out the evidences of macular pathology. In this regard, those with a history of diabetes mellitus, neuromuscular disorders, reaction to anti-malaria drugs, uncontrolled hypertension or history of retinopathy, decision on any surgical intervention, treatment with digoxin or corticosteroids, history of psoriasis, history of chronic bowel diseases or history of malignancy were not included. All patients were explained the details of the study protocol. The ethics committee at the Isfahan University of Medical Sciences approved the study protocol.
Figure 1.
Consort chart for the study
Procedures and variable assessment
Initially, the blood sample was obtained from all participants after a 12-hour fasting to examine biochemical markers including fasting blood sugar, OGTT, hemoglobin A1c, lipid profile, blood count, blood urea nitrogen, creatinine, liver enzymes, C-reactive protein, fasting insulin by the immunoassay method (Simense kit, Tarrytown, NY, USA)) and also insulin level after OGTT test. In this context, those patients with fasting blood sugar level 100–125 mg/dL or hemoglobin A1c 5.7–6.5% were included in the study. All patients were physically examined by the physician and anthropometric parameters such as weight, height, body mass index and waist circumference were measured. The participants were then randomly assigned using a computer-generated randomized list to receive hydroxychloroquine (6.5 mg/kg/day) (n = 20) or placebo (n = 19) for 12 weeks. After completing the treatment period, the biomarker indices and anthropometric parameters were tested again in both groups.
Statistical analysis
Results are presented as mean ± standard deviation (SD) for quantitative variables and were summarized by frequency (percentage) for categorical variables. Continuous variables were compared using the t test or the Mann–Whitney U test whenever the data had normal distribution or not. The within-group comparisons were examined by the paired t-test or the Wilcoxon signed rank test. Categorical variables were, on the other hand, compared using the Chi-square test. For evaluating the changes in serum glucose in the studied groups, repeated measure ANOVA was used. For the statistical analysis, the statistical software SPSS version 20.0 for windows (SPSS Inc., Chicago, IL, USA) was used. P values of 0.05 or less were considered statistically significant.
RESULTS
The two groups were matched for age and gender. The mean age of patients in the hydroxychloroquine and placebo groups was 45.90 ± 7.32 years and 46.79 ± 6.35 years (P = 0.688) and the frequency of male gender was 25.0% and 36.8% (P = 0.423), respectively. Table 1 shows changes in study parameters following interventional protocols [Table 1]. In both groups of patients receiving hydroxychloroquine and placebo, except for the serum level of insulin that was significantly elevated after treatment with hydroxychloroquine, the changes in the other parameters remained insignificant [Table 2]. Although both groups experienced an increase of insulin level (from 12.3 ± 10.6 to 78.3 ± 53.5 units in case group and from 9.8 ± 5.3 to 40.8 ± 31.4 unit in control group), but this change was considerably higher in those patients receiving hydroxychloroquine (P = 0.009). Changes in blood glucose after OGTT test before intervention have shown in Figure 2. Also, regarding changes in serum glucose concentration, there were no significant differences in the changes of blood glucose level after intervention in each group [Figure 3]; however, the group of patients receiving hydroxychloroquine experienced reduction of glucose at 60 min of OGTT test after intervention, while the placebo group experienced increase of blood glucose at the same time (from 163.0 ± 39.4 to 158.4 ± 36.3 mg/dL in the case group and from 163.3 ± 39.2 to 181.7 ± 51.7 mg/dL in the control group, P = 0.033).
Table 1.
Changes in study parameters following interventional protocols
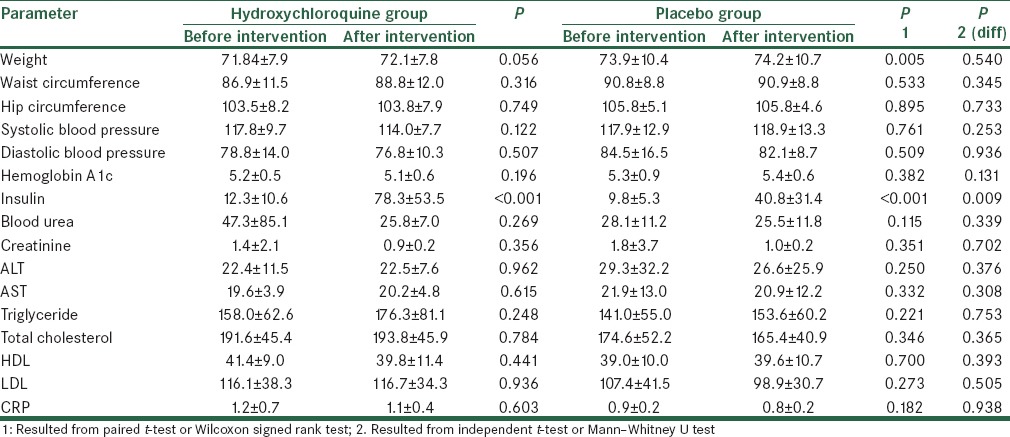
Table 2.
Changes in blood glucose at different time points following interventional protocols
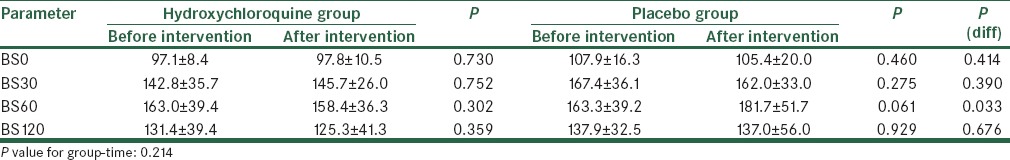
Figure 2.
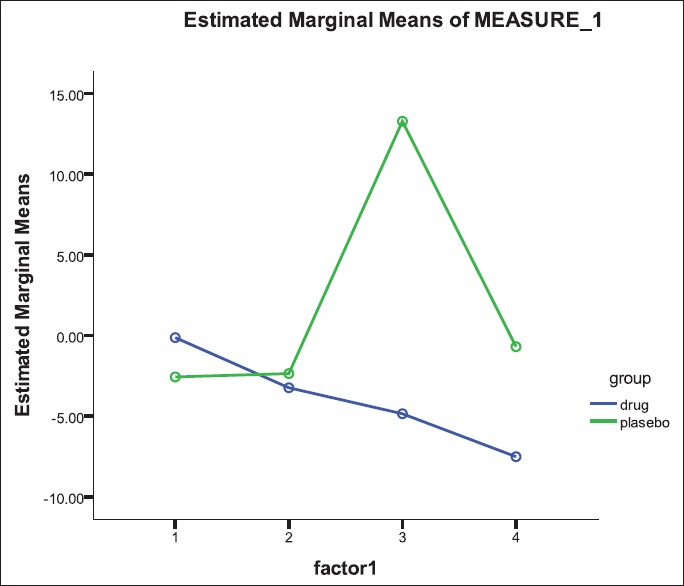
Changes in blood glucose after OGTT test before intervention
Figure 3.
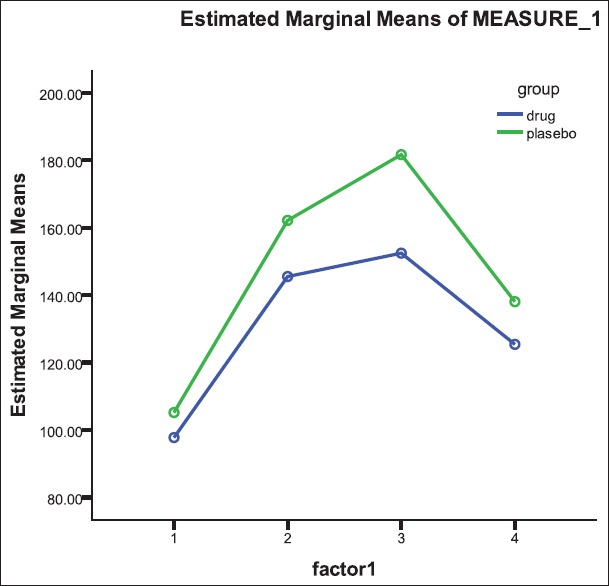
Changes in blood glucose after OGTT test after intervention
DISCUSSION
This clinical interventional study demonstrated that 12 weeks of hydroxychloroquine treatment reduced the serum glucose levels of the patients studies. Several studies have shown that hydroxychloroquine positively affects insulin and glucose metabolism.[4,7,8,10,11,12,13] In fact, the highlighted point of the present study was the effects of hydroxychloroquine on glucose control in the prediabetes, state independent of its effect on body weight and composition as well as on the lipid profile. In fact, the hypoglycemic effect of hydroxychloroquine has been identified as its potential side-effect, but because of no another concomitant effects on other metabolic components and body organs, this effect can be beneficially used for controlling glucose and also for progression of prediabetes to diabetes state. In this regard, the use of 6.5 mg/kg/day hydroxychloroquine for 12 weeks seems to be suitable for this aim. The main mechanism for the effect of hydroxychloroquine has not been fully clarified until now. One suggested hypothesis is reflected by a decreased degradation rather than increased secretion of insulin. Quatraro and colleagues[14] demonstrated reduced insulin requirements in type II diabetic patients without altered C-peptide levels, thereby suggesting unaltered secretion of insulin. Moreover, decreased insulin resistance has also been suggested to contribute to the same, as demonstrated for patients with systemic lupus erythematosus.[10] A large prospective cohort study among rheumatoid arthritis patients showed that use of hydroxychloroquine was associated with decreased risk of type II diabetes mellitus development.[6] Although we did not find any changes in any other cardiovascular risk profile, including lipid profile or obesity, it has been shown that the cardiovascular risk is diminished by hydroxychloroquine usage with a more beneficial lipid profile and decreased diastolic blood pressure.[15]
Despite beneficial effects of hydroxychloroquine on insulin sensitivity and diabetes control, monitoring and controlling its dosage is very critical. Although long-term use of chloroquine is associated with congestive heart failure and rhythm disturbances, hydroxychloroquine can be considered safe in appropriate doses as applied in our trial,[16] even if risk factors like renal failure, high-dose therapy and preexisting cardiac disease may be present in higher doses or in longer treatment periods.[17] For instance, in those patients who receive digoxin therapy, minute caution is needed because of the interaction between these drugs. In this context, the use of this drug is preferred in those hyperglycemic patients with noncardiovascular conditions.
In this study, despite an increase in the serum insulin level, the glucose tolerance remained unchanged. Some authors concluded that both groups with normal glucose tolerance and impaired glucose tolerance had the same relative increase in insulin levels after oral glucose, indicating an equivalent beta-cell function.[18] It seems probable that the observed effect can be induced by the incretin effect, which enhances insulin response leading to heterogeneity of insulin responses during OGTT.[19,20]
In conclusion, hydroxychloroquine can effectively lower glucose levels through increase insulin level. Clinicians should be warranted about hypoglycemia during hydroxychloroquine usage and consider its controlled dosage and treatment period. Treatment with hydroxychloroquine can be employed as a therapeutic in patients with prediabetic states who are at risk of developing diabetes mellitus.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Haffner SM. Pre-diabetes, insulin resistance and CVD risk. Diabetic Res Clin Pract. 2003;61:S9–18. doi: 10.1016/s0168-8227(03)00122-0. [DOI] [PubMed] [Google Scholar]
- 2.Bock G, Dallamana C, Campioni M, Chittilepilly E, Basu R, Toffolo G, et al. Pathogenesis of Pre- Diabetes. (Mechanisms of Fasting and post prandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose Tolerance. Diabetes. 2006;55:3536–49. doi: 10.2337/db06-0319. [DOI] [PubMed] [Google Scholar]
- 3.Osei K, Galland T, hinsmith S. Impaired Insulin Sensivity, Insulin Secretion, and Glucose Effectivness Perdic Future Develipment of Impaired Glucose and Glucose Tolerance and type 2 diabetes in pre-diabetic African – Americans. Diabet Care. 2004;27:1439–46. doi: 10.2337/diacare.27.6.1439. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Thorpe KE, Taylon DW, Haynes RB. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas – A randomized trial. Diabetes Res Clin Pract. 2002;55:209–19. doi: 10.1016/s0168-8227(01)00325-4. [DOI] [PubMed] [Google Scholar]
- 5.Emami J, Gerstein HC, Pasutto FM, Jamali F. Insulin – Sparing effect of hydroxychloroquine in diabetic rats is concentration dependent. Can J Physiol Pharmacol. 1999;77:118–23. [PubMed] [Google Scholar]
- 6.Wasko MC, Hubert HB, Lingala VB, Elliott JR, Luggen ME, Fries JF, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187–93. doi: 10.1001/jama.298.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Qatraro A, Consoli G, Magno M, Caretta F, Nardozz A, Ceriello A. Hydroxychloroquin in decompensated treatment – Refractory non insuli – Dependent diabetes mellitus, A new job for an old drug.? Ann Intern Med. 1990;112:628–81. doi: 10.7326/0003-4819-112-9-678. [DOI] [PubMed] [Google Scholar]
- 8.Petri M, Lakatta C, Magder L, Goldman D. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: A longitudinal data analysis. Am J Med. 1994;96:254–9. doi: 10.1016/0002-9343(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 9.Morris SJ, Wasko MC, Antohe JL, Sartorius JA, Kirchner HL, Dancea S, et al. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2011;63:530–4. doi: 10.1002/acr.20393. [DOI] [PubMed] [Google Scholar]
- 10.Penn SK, Kao AH, Schott LL, Elliott JR, Toledo FG, Kuller L, et al. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 2010;37:1136–42. doi: 10.3899/jrheum.090994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525–31. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 12.Emami J, Gerstein HC, Pasutto FM, Jamali F. Insulin-sparing effect of hydroxychloroquine in diabetic rats is concentration dependent. Can J Physiol Pharmacol. 1999;77:118–23. [PubMed] [Google Scholar]
- 13.Garcia-Webb P, Bonser AM. Insulin binding and degradation in isolated hepatocytes from streptozotocin injected rats. Biochem Biophys Res Commun. 1985;128:487–93. doi: 10.1016/0006-291x(85)90073-7. [DOI] [PubMed] [Google Scholar]
- 14.Quatraro A, Consoli G, Magno M, Caretta F, Nardozza A, Ceriello A, et al. Hydroxychloroquine in decompensated, treatment-refractory noninsulin-dependent diabetes mellitus. A new job for an old drug? Ann Intern Med. 1990;112:678–81. doi: 10.7326/0003-4819-112-9-678. [DOI] [PubMed] [Google Scholar]
- 15.Rho YH, Oeser A, Chung CP, Milne GL, Stein CM. Drugs Used in the Treatment of Rheumatoid Arthritis: Relationship between current use and Cardiovascular Risk Factors. Arch Drug Inf. 2009;2:34–40. doi: 10.1111/j.1753-5174.2009.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costedoat-Chalumeau N, Hulot JS, Amoura Z, Delcourt A, Maisonobe T, Dorent R, et al. Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology. 2007;107:73–80. doi: 10.1159/000094079. [DOI] [PubMed] [Google Scholar]
- 17.Costedoat-Chalumeau N, H ulot JS, Amoura Z, Leroux G, Lechat P, Funck-Brentano C, et al. Heart conduction disorders related to antimalarials toxicity: An analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology (Oxford) 2007;46:808–10. doi: 10.1093/rheumatology/kel402. [DOI] [PubMed] [Google Scholar]
- 18.Nauck MA, Sauerwald A, Ritzel R, Holst JJ, Schmiegel W. Influence of glucagon-like peptide 1 on fasting glycemia in type 2 diabetic patients treated with insulin after sulfonylurea secondary failure. Diabetes Care. 1998;21:1925–31. doi: 10.2337/diacare.21.11.1925. [DOI] [PubMed] [Google Scholar]
- 19.Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159–69. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- 20.Rekedal LR, Massarotti E, Garg R, Bhatia R, Gleeson T, Lu B, et al. Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Rheum. 2010;62:3569–73. doi: 10.1002/art.27703. [DOI] [PMC free article] [PubMed] [Google Scholar]