Fig.7.
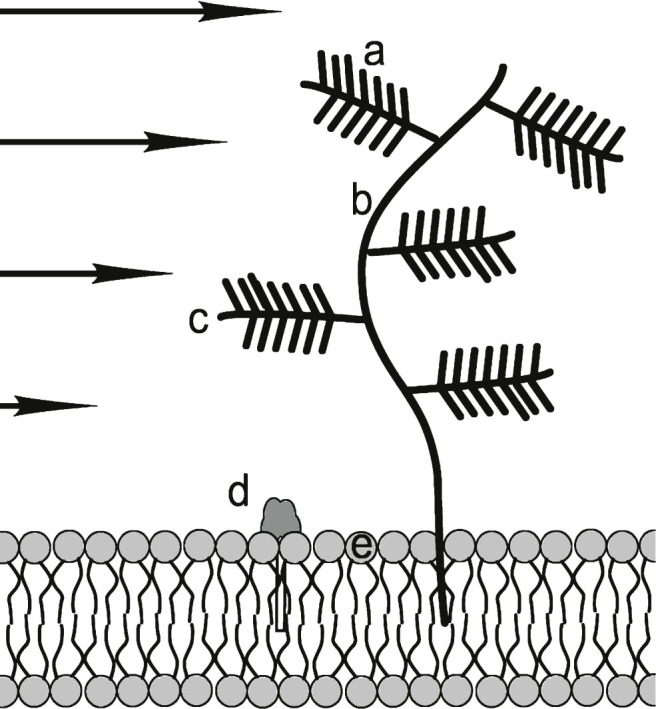
Heparan sulfate proteoglycan (HSPG) consisting of: a) negatively charged sulfate compound “needles”; b) main branch hyaluronan; c) protein side branch; d) polar head of GM1 ganglioside that is reported to bind to amyloid oligomers: e) polar membrane phospholipid head. Negatively charged HSPG molecules attract positively charged Aβ side chains, if they are not internally bound. It is proposed that in sheared Aβ* they are HSPG accessible. GM1 molecules are rich in hydrophilic –OH groups that are attractive to Aβ and Aβ* groups. In addition, HSPG “trees” interfere with ISF flow and presents a flow obstacle. Other molecular flow obstructions such as laminin and collagen are not shown. Arrows indicate differential shear flow near the membrane. Figure not to scale.
