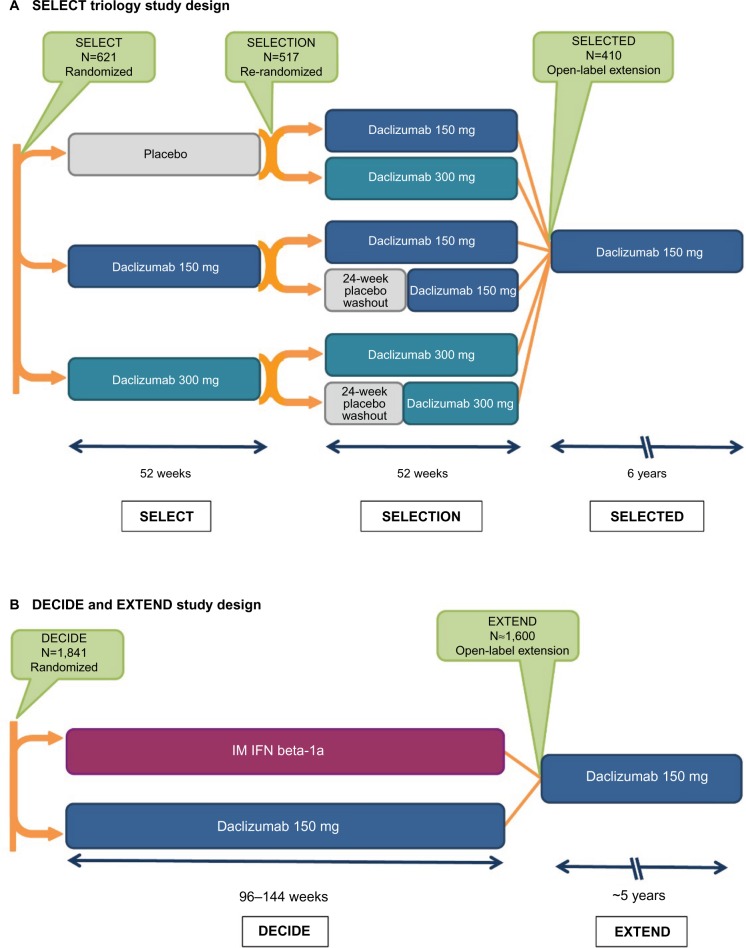
Figure 1.
The SELECT and DECIDE studies and their extension studies.41,42,51–53,129
Notes: (A) In SELECT, patients with RMS were randomized to receive daclizumab 150 or 300 mg subcutaneously every 4 weeks or placebo for 52 weeks.41 Patients who completed SELECT were eligible to enroll in the SELECTION extension study. In SELECTION, patients from the placebo group in SELECT were re-randomized to receive daclizumab 150 or 300 mg subcutaneously every 4 weeks for 52 weeks; patients from the daclizumab 150 and 300 mg treatment arms in SELECT were re-randomized to continue their assigned dosage of daclizumab or to 20 weeks of placebo treatment followed by reinitiation of their previously assigned dosage of daclizumab for 32 weeks.52 In the placebo washout group, the period of time between the last daclizumab dose in SELECT and reinitiation of daclizumab in SELECTION was a total of 24 weeks.52 Patients who completed SELECTION were eligible to enroll in the ongoing open-label SELECTED study in which all patients received daclizumab 150 mg for up to 6 years.53,129 (B) In DECIDE, patients were randomized to receive daclizumab 150 mg subcutaneously every 4 weeks or IFN beta-1a 30 mcg IM once weekly for a minimum of 96 weeks up to a maximum of 144 weeks; the study ended when the last enrolled patient completed the week 96 visit.42 Patients who completed DECIDE were eligible to enroll in the ongoing EXTEND safety extension study in which all patients received daclizumab 150 mg for up to 6 years of total time on treatment.51
Abbreviations: IM, intramuscular; IFN, interferon; RMS, relapsing multiple sclerosis.