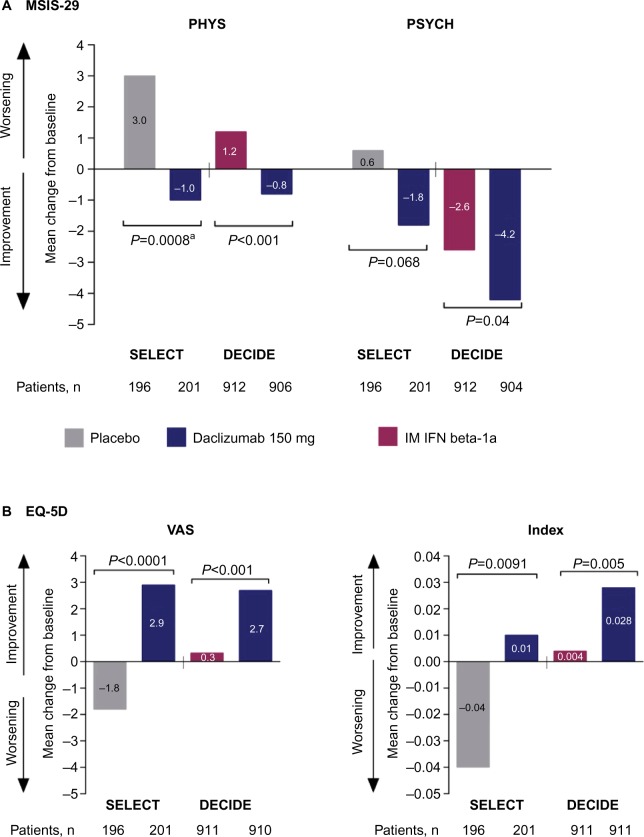
Figure 3.
Changes from baseline to weeks 52 and 96 in health-related quality-of-life endpoints in SELECT and DECIDE.41,42
Notes: aNominal P-value. Comparison was not considered statistically significant within the sequential closed testing procedure for secondary endpoints.41 (A) Change from baseline to week 52 or 96 in MSIS-29 PHYS and PSYCH subscale scores. (B) Change from baseline to week 52 or 96 in EQ-5D VAS and Index scores. In both the DECIDE and SELECT studies, between treatment group differences in MSIS-29 PHYS and PSYCH scores and EQ-5D VAS and index scores were evaluated using analysis of covariance models adjusting for baseline factors. The statistical models are detailed in the original articles.41,42 SELECT data adapted with permission from Elsevier (The Lancet, 2013, 381, 2167–75).
Abbreviations: MSIS-29, 29-item Multiple Sclerosis Impact Scale; PHYS, physical impact subscale; PSYCH, psychological impact subscale; IM, intramuscular; IFN, interferon; EQ-5D, EuroQol 5-Dimensions; VAS, visual analog scale.