Table 2.
Reactions between benzyne and other enol ethers or vinylene carbonate.[a]
| Entry | Reactant | Product | Time (h), yield |
|---|---|---|---|
| 1 |
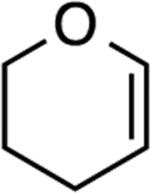
|
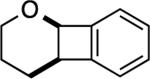
|
2: 2 h, 72% |
| 2 |
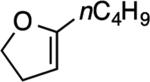
|
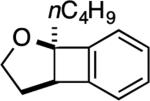
|
3: 6 h, 69% |
| 3 |
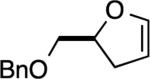
|
|
4a, 4b: 3 h, 48% 1:1 mixture |
| 4 |
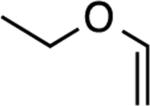
|
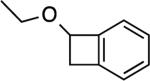
|
5: 1 h, 50%[b] |
| 5 |
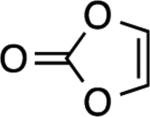
|
NA[c] | – |
| 6 |
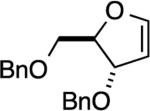
|
NA[c] | – [d] |
| 7 |
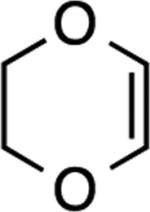
|
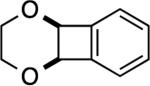
|
6: 2 h, 84% |
Reactions were conducted at a 0.125 m concentration of 2-(trimethylsilyl)phenyl triflate in MeCN, in the presence of 1.2 equiv. of the enol ether (or vinylene carbonate), 2 equiv. of CsF, and 2 equiv. of 18-Cr-6 at room temperature.
Yield obtained with 3 equiv. of ethyl vinyl ether.
n.a.: not applicable.
About 86% of the glycal was recovered.
