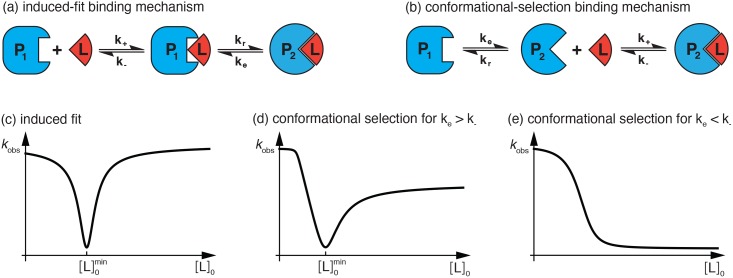
Fig 1. Characteristic chemical relaxation of induced-fit and conformational-selection binding.
(a) In induced-fit binding, the change between the conformations P1 and P2 of the protein occurs after binding of the ligand L. The intermediate state P1L relaxes into the bound ground state P2L with rate kr, and is excited from the ground state with rate ke. (b) In conformational-selection binding, the conformational change of the protein occurs prior to ligand binding. The intermediate state P2 is excited from the unbound ground state P1 with rate ke, and relaxes back into the ground state with rate kr. (c) The dominant, smallest relaxation rate kobs of induced-fit binding is minimal at the total ligand concentration where [P]0 is the total protein concentration and Kd the overall dissociation constant. As a function of [L]0, the dominant rate kobs is symmetric with respect to this minimum. (d) The dominant, smallest relaxation rate kobs of conformational-selection binding has a characteristic minimum as a function of [L]0 for ke > k−, but is not symmetric with respect to this minimum. (e) The dominant rate kobs of conformational-selection binding decreases monotonically with [L]0 for ke < k−.