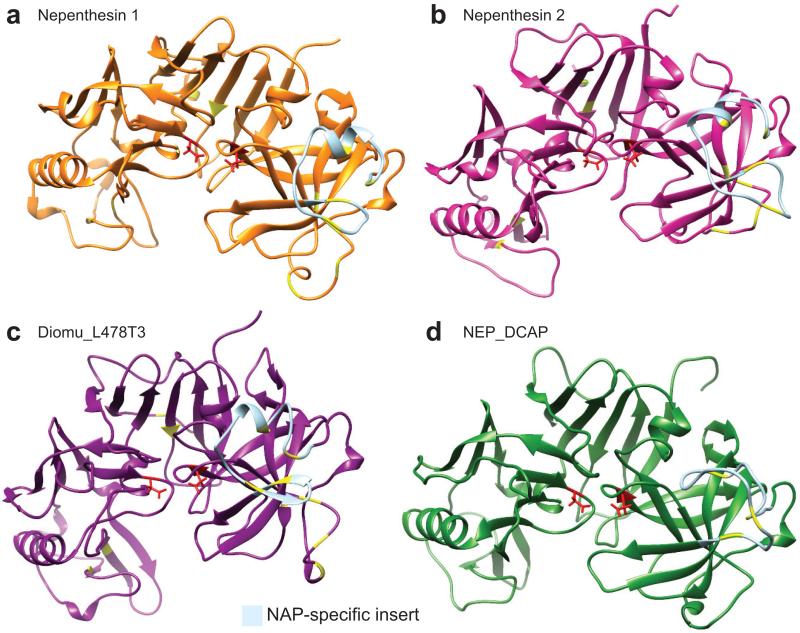
Figure 7.
Nepenthesin structure predictions. Three-dimensional structure prediction of nepenthesins 1 and 2 from N. gracilis (a and b), Diomu_L478T3 from D. muscipula (c), and NEP_DCAP from D. capensis (d). The active site Asp residues are highlighted in red, while the cysteines believed to be involved in disulfide bonds (by homology to nepenthesin 1) are shown in yellow. The NAP-specific insert, which is not removed during processing and contributes to the enhanced stability of the nepenthesins, is colored light blue.