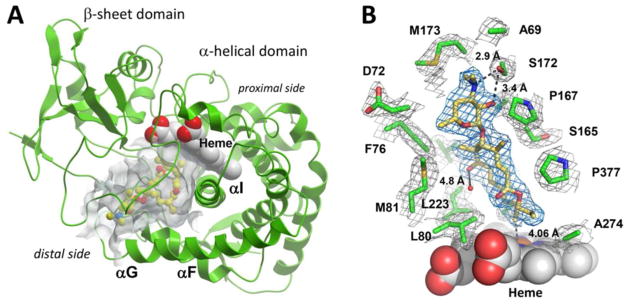
Figure 4.
Crystal structure of MycCI with 9 bound in the active site. (A) The protein scaffold is represented by green ribbons. 9 (ball-and-stick mode: yellow = carbon, red = oxygen, blue = nitrogen) occupies the 7000 Å3 pocket (semi-transparent grey surface) of the α-helical domain interfacing the β-sheet domain. Image was generated using the MOLSOFT ICM-Browser.77 (B) A fragment of the 2Fo−Fc electron density map contoured at 1 σ delineates 9 (blue mesh) and a set of amino acid residues (green) within 4 Å of 9 (grey mesh). Distances reported are in Ångströms. The heme cofactor is depicted as van der Waals spheres (grey = carbon, red = oxygen, blue = nitrogen, ochre = iron). The water molecule present in both chains A and B of the asymmetric unit is shown as a small red sphere. Image was prepared using PyMOL.78