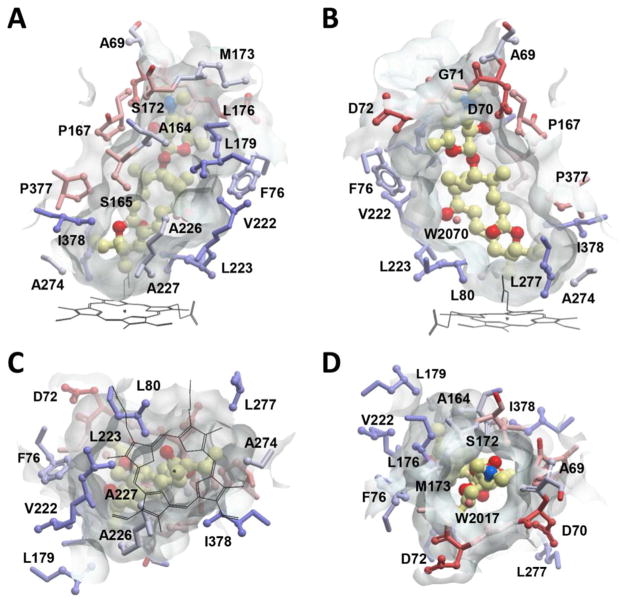
Figure 5.
The binding site of 9 in MycCI, represented by a semi-transparent surface surrounding the substrate molecule (ball-and-stick mode: yellow = carbon, red = oxygen, blue = nitrogen). Amino acid residues are in ball-and-stick mode and are colored by hydrophobicity in a color gradient ranging from ice-blue (hydrophobic) to red (hydrophilic). Water molecules are shown as small spheres. Images were generated using the MOLSOFT ICM-Browser.77 (A) Side view of the binding site showing the hydrophobic wall built by lipophilic amino acids. (B) The opposite side of the binding pocket facing the β-sheet domain is more polar and loosely built. (C) Bottom view of the binding site with the heme cofactor (black lines) projected onto the semi-transparent surface. Six lipophilic residues along the edge of the heme macrocycle (L80, L223, A227, A274, L277, and I378) form a pocket that surrounds the end of the M-VIII macrolactone opposite desosamine, thus positioning the C21 methyl group above the iron center. (D) Top view along the axis of 9 with the desosamine moiety visible through the surface opening.