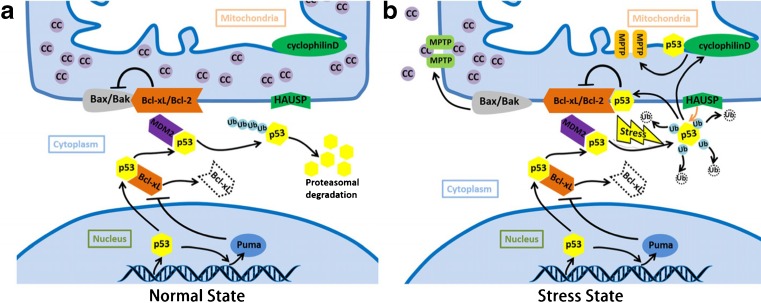
Fig. 2.
p53 induced apoptosis and necrosis. a The molecules and pathway in mitochondrial p53 translocation in normal state. After translation, Bcl-xL will bind to p53 and inhibits its function. Nuclear p53 transactivates its target gene Puma and induce the formation of Puma/Bcl-xL complex, which could liberate p53 in cytoplasm. MdM2 contributes to the monoubiquitination of p53, leading into the p53 degradation. b The molecules and pathway in mitochondrial p53 translocation in stress state. After the stress stimuli, mitochondrial p53 accumulation can be detected. Monoubiquitinated p53 can change into polyubiquitination state. Polyubiquitinated p53 can switch to deubiquitination state and translocate to the outer mitochondrial membrane, neutralizing the inhibitory function of Bcl-2/Bcl-xL in the presence of HAUSP. Bax and Bak can then be released and form the MPTP, leading to the cytochrome c release, and apoptosis finally. Besides, deubiquitination p53 can decrease inner membrane potential. p53 can bind to cyclophilin D on inner membrane, activated cyclophilin D contributes the inner membrane MPTP as well, leading into the necrosis finally. Abbreviations: cc: cytochrome c, MPTP: mitochondrial permeability transition pore. HAUSP: herpesvirus associated ubiquitin specific protease, Bcl-2: B-cell lymphoma 2, Bcl-xL: B-cell lymphoma-extra-large (BCL2-like 1), Bak: Bcl-2-homologous antagonist/killer, Bax: Bcl-2-associated X protein, Puma: p53 upregulated modulator of apoptosis, MDM2: Murine Double Minute 2