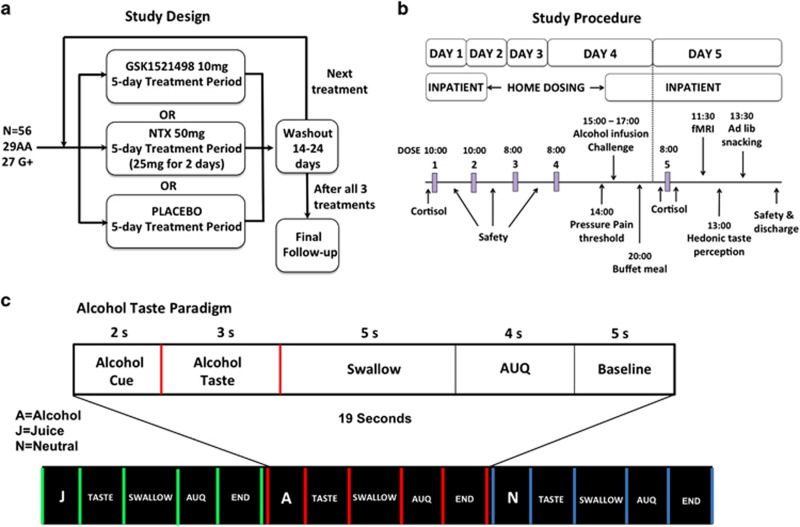
Figure 1.
Overview of study design (a) and study procedures (b). In each treatment period, participants were admitted to the GSK Clinical Unit Cambridge (CUC) on day 1 for an overnight stay. They underwent safety assessments, routine blood tests, and received their first dose (GSK1521498 10 mg, NTX 25 mg, or placebo). On day 2, participants received their second dose, repeated safety assessments, and were discharged with medication doses for days 3 and 4. They returned to the CUC on the afternoon of day 4 and underwent pain threshold assessments, an intravenous alcohol infusion challenge, and were served a buffet dinner. After an overnight fast, they received their final dose on day 5, then underwent fMRI scanning followed by hedonic taste response tests and an ad libitum snacking paradigm. Participants were discharged at the end of day 5 after final safety assessments. Participants attended a follow-up assessment 7–10 days after completion of the final washout period. (c) Overview of fMRI taste paradigm and trial structure.