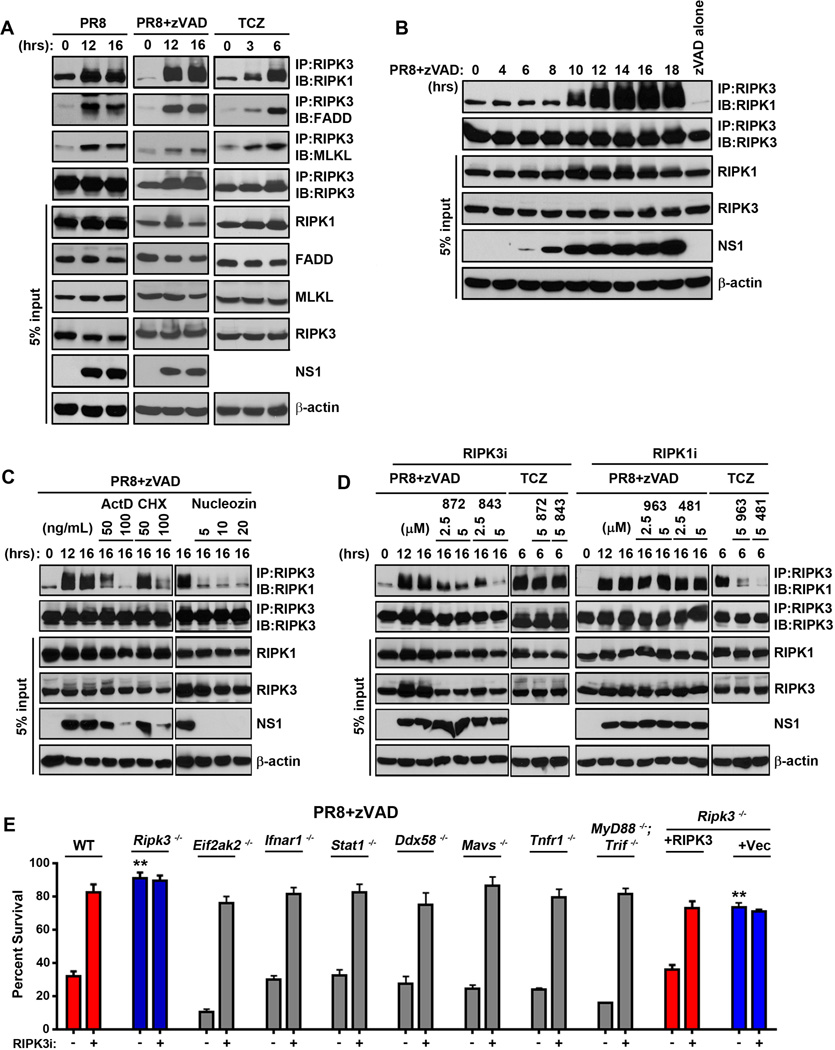
Figure 2. IAV activates formation of a RIPK3-containing necrosome complex.
(A) Wild type MEFs were infected with PR8 (m.o.i.=2) in the presence or absence of zVAD (25µM), or treated with TCZ (right). Cells were lysed at the indicated time points, and anti-RIPK3 immunoprecipitates were examined for necrosome formation as described before (Thapa et al., 2013). IAV- or TCZ-activated RIPK3-RIPK1 necrosomes additionally contain FADD and MLKL. Whole-cell extract (5% input) was examined in parallel for RIPK1, RIPK3, FADD, MLKL, and IAV NS1 proteins. (B) Time course of RIPK1/RIPK3 necrosome formation demonstrates that necrosome assembly succeeds virus replication. (C) RIPK3 immunoprecipitations performed on extracts from PR8-infected MEFs that were pre-treated with the indicated doses of Actinomycin D (ActD), cycloxehimide (CHX) or Nucleozin (Nuc), before infection indicates that the IAV-induced necrosome formation requires ongoing transcription, translation, and viral replication. (D) RIPK3 immunoprecipitations were performed on extracts from MEFs infected with PR8 or treated with TCZ in the presence or absence of the indicated doses of the RIPK3 kinase inhibitors GSK’872 or GSK’843 (left) or the RIPK1 kinase inhibitors GSK’963 or GSK’481 (right). (E) Primary early passage wild-type (WT) and the indicated knock-out MEFs were infected with PR8 in the presence of zVAD (50µM) with or without the RIPK3 inhibitor GSK’843 (5µM). As controls, ripk3−/− MEFs retrovirally reconstituted with full-length murine RIPK3, or with an empty vector, were used. Viability was determined 24 h.p.i. Error bars represent mean +/− S.D. ** p <0.005. See also Figs. S3 and S6.