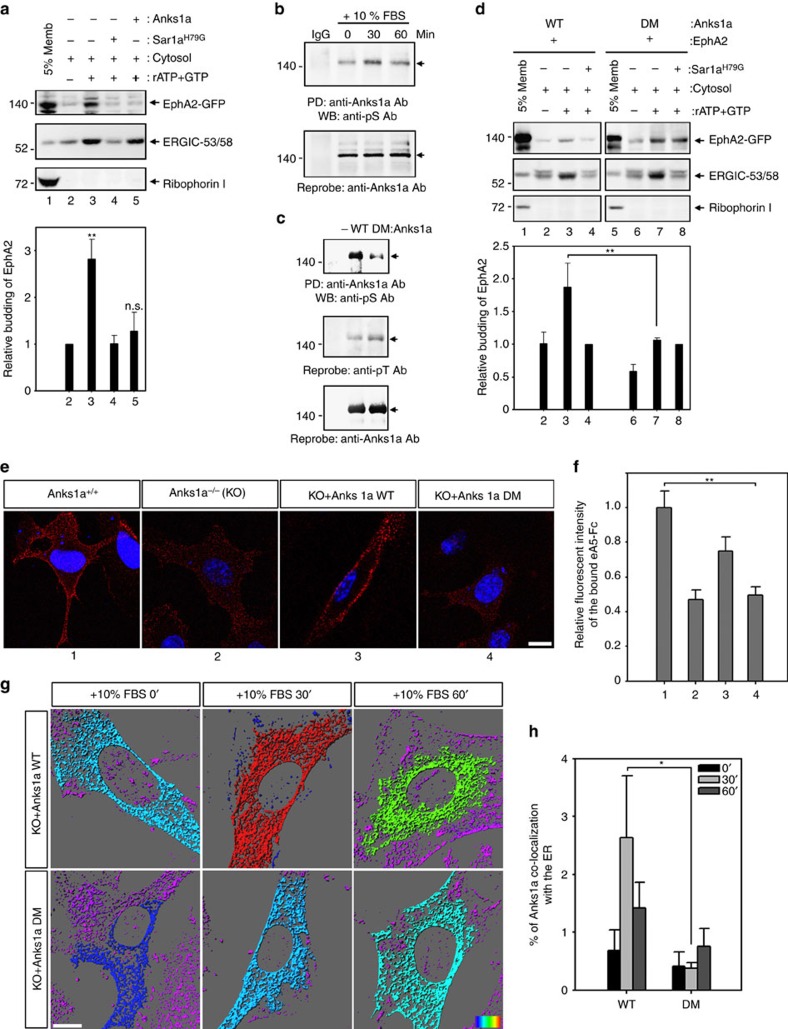
Figure 3. Serine phosphorylation of Anks1a is important for the ER export of EphA2.
(a) Experiments were performed essentially as in Fig. 2f, except that purified Anks1a protein (His tagged) was added to the budding assay (n=4). **P<0.01, one-way analysis of variance (ANOVA) test. (b) Serum-starved CT26 cells were stimulated with 10% FBS for the indicated times. Each cell lysate was incubated with an anti-Anks1a antibody and the bound proteins were detected with an anti-phosphoserine (pS) antibody (top panel). (c) HEK293T cells were transfected with the indicated constructs and experiments were performed as described in b. Note that serine residues 647 and 663 were replaced with alanine in the Anks1a-DM mutant. (d) Experiments were performed as described in Fig. 2f except for the substitution of the indicated Anks1a constructs. It is unclear why addition of the ATP regeneration system induces COPII-independent budding of EphA2 in Anks1a-DM-transfected cells (top panel, lanes 7 and 8). This necessitated the use of Sar1aH79G as a control for the normalization of EphA2 budding in other conditions (n=4). (e,f) Experiments were performed essentially as described in Figure 1i. Scale bar, 10 μm. **P<0.01, one-way ANOVA test. (g,h) Anks1a knockout (KO) MEF cells were transfected with the indicated Anks1a constructs. Forty-eight post transfection, cells were starved for 2 h and stimulated with 10% FBS for the indicated times. Then, the cells were immunostained with both anti-Anks1a and anti-Calregulin antibodies. Co-localization in each panel is presented in a pseudo-coloured heat map. Co-localization efficiency of Anks1a with ER was calculated using Imaris (n=15 for each case). Scale bar, 10 μm. *P<0.05, Student's t-test. n.s., not significant. Data represent means±s.e.